England. A world superpower. Not just in everything else, but also in science, which is world-beating. English spitfire pluckiness destroyed the Huns in two world wars and in Brexit, but also in scientific performance, both impact-factor and money-wise. Brexit Britain will be free of all diseases, because whatever an English scientist does, conclusions remain not affected.
The secret to English science supremacy is not just the limitless capacity of certain English researchers to fabricate exciting results and phony cures, anyone can do that. It is the unique arrogance and corruption of English universities and of British health authorities who regularly decree all data fakeries to be quality science, outside statute of limitations, and verified independently by many citations, while telling whistleblowers to get lost.
It is a game only the crooks can win. And tax payers, charity donors and patients pay the price.
Not matter what you hear from Brexit, it’s not like England is not tolerant and welcoming. Clean of foreign detritus, free Britain can now freely choose what kind of foreigner it will let it. The foreigner must bring the right skill, like Amato Giaccia did, when he arrived to Oxford from Stanford in USA, trailed by a string of questionable research. Brexit and the COVID-19 pandemic showcased what the English ruling elites value most: defrauding and bullshitting the public. Science is just another coffer to plunder in this scam.
I previously celebrated the English science genius, with superior English minds like regenerative medicine enthusiast Martin Birchall, who took over Paolo Macchiarini‘s deadly technology to run some trachea transplants of his own. Birchall was acquitted in full by his own UCL, including for data irregularities, foreign scapegoats were assigned. Also Master of Birkbeck Sir David Latchman, CBE, was saved by UCL from unruly investigators whose reports were overruled by officials to determine no misconduct. Dame Kay Davies, DBE, was defended by her University of Oxford thanks to statute of limitations of 3 years for research misconduct charges, and by the publisher Oxford University Press where she as Editor-in-Chief retaliated against a fraud whistleblower. Paul Workman and Alan Ashworth, current and former director of ICR London, were both absolved in full, nothing at all worth retracting was found, while minor characters (not their protegees though) got the blame. Richard Marais of Cancer Research UK in Manchester weathered a research fraud scandal in his lab and a mountain of bullying accusations, he is still director of the institute.
Now, I shall celebrate more such talents, based on the sleuthing of Clare Francis. Cancer is cured, brain diseases vanquished, all thanks to the English scholars I want you to admire. Please raise, tuck in your shirt and comb your hair. The honour is all yours:

Nick Lemoine, FRCPath FMedSci
We shall start with Nicholas Lemoine. This cancer researcher is professor at the Queen Mary University of London and director of the Cancer Research UK Barts Centre. He is also an extremely high-ranking expert at the MRC (Medical Research Council), which means Dr Nick decides which biomedical researchers in UK gets funded and which don’t:
“He has served as Chair of the Clinical Training and Career Development Panel at the Medical Research Council, and as Vice-Chair of the MRC Stratified Medicine Expert Panel. He has previously served as Chair of the MRC Stem Cell Strategic Grant and Fellowship Panels, and has been a member of the MRC’s Molecular & Cellular Medicine Board.“
As medical director of NIHR Clinical Research Network, Dr Nick is now in charge of UK’s COVID-19 response:
So what kind of science are you, a humble British scientist seeking to eke out a meagre grant from MRC or NIHR, expected to deliver in order to impress Professor Lemoine? Well, you could study from his own papers, here is one:
Iman El-Hariry, Massimo Pignatelli, Nicholas R. Lemoine FGF-1 and FGF-2 regulate the expression of E-cadherin and catenins in pancreatic adenocarcinoma International Journal of Cancer (2001) DOI: 10.1002/ijc.1515
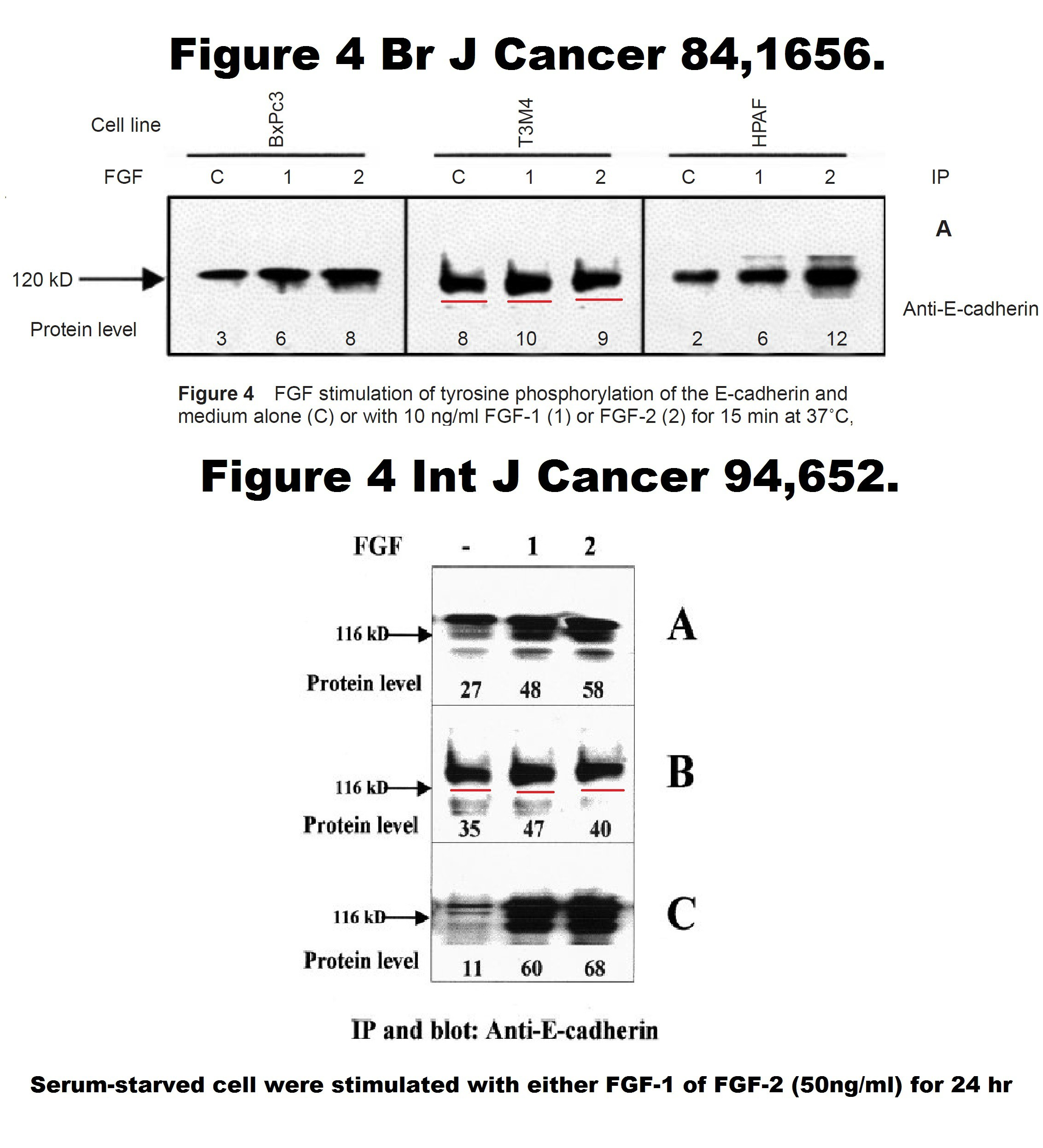
As you see, the Figure 4 is fake, it has 3 cloned bands. Which means, the conclusions are not affected. A correction was issued by the publisher Wiley in 2019:
“There is an error in the experimental conditions reported for the data presented in Figure 4 panel B. We apologize for these circumstances, which required clarification of the data.”
As it happens, Ashworth’s mentee and first author on a bunch of hilariously fake papers, Sarah Martin, is presently faculty member and Deputy Centre Lead at the Lemoine-ruled Bart’s and, this is really cruel: director of the graduate school. Now you see which qualifications you need to get employed at Barts, plus they probably sack PhD students for refusing to fake data there.
Maybe they use this paper by Limoine and his Barts colleagues to teach the students how to do cancer research properly:
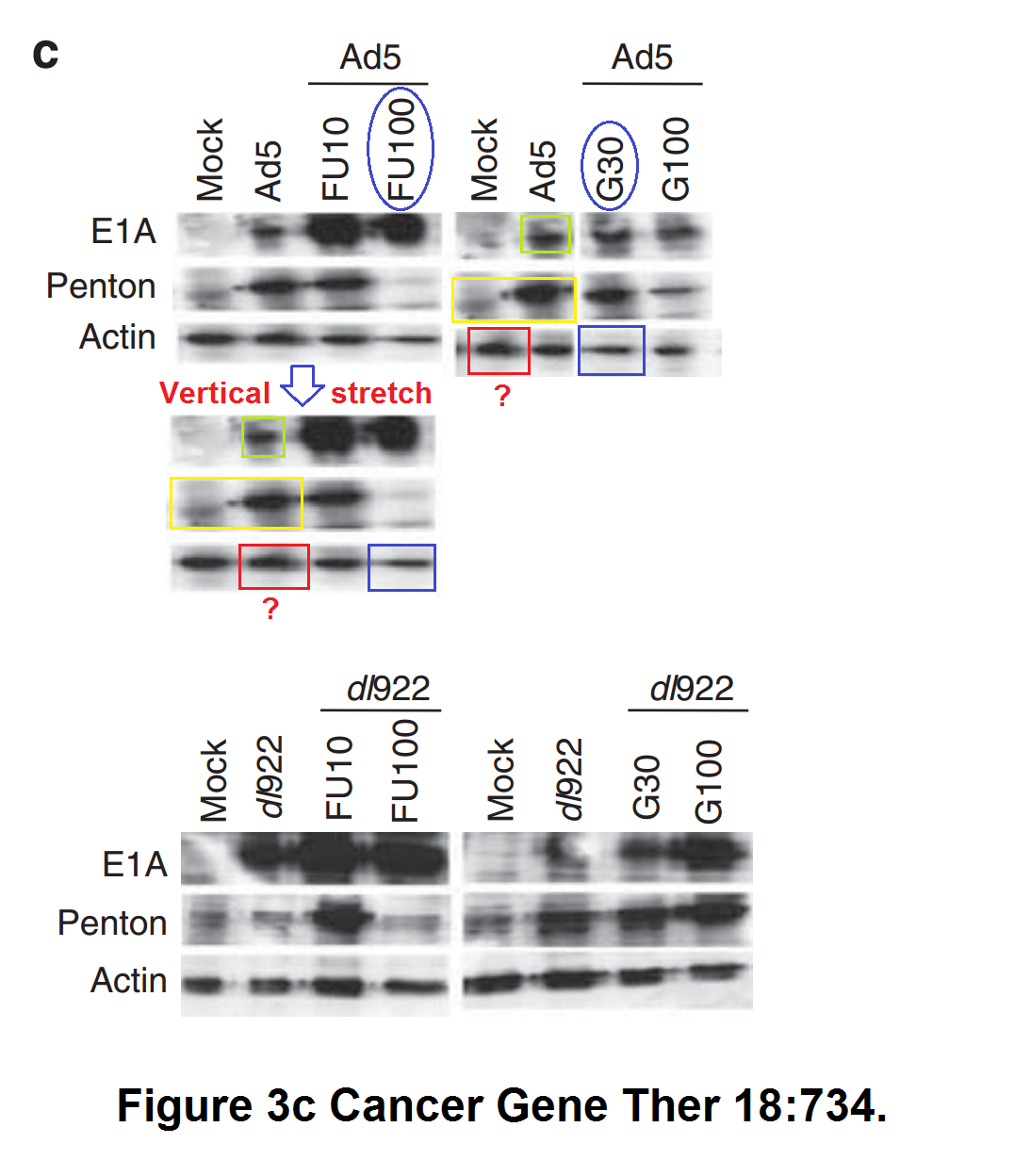
M Bhattacharyya, J Francis, A Eddouadi, N R Lemoine, G Halldén An oncolytic adenovirus defective in pRb-binding (dl922–947) can efficiently eliminate pancreatic cancer cells and tumors in vivo in combination with 5-FU or gemcitabine Cancer Gene Therapy (2011) doi: 10.1038/cgt.2011.45
Nothing was done. The Editor-in-Chief of this Nature Research Group journal is another professor in England, Georgios Giamas, owner of his own PubPeer record, and protege of the controversial, yet perfectly safe in his job, Imperial College professor Justin Stebbing. Giamas also holds a visiting professorship at Imperial, and he will definitely never do anything about the following paper by Limoine in Cancer Gene Therapy:
I A McNeish, T Tenev, S Bell, M Marani, G Vassaux, N Lemoine Herpes simplex virus thymidine kinase/ganciclovir–induced cell death is enhanced by co-expression of caspase-3 in ovarian carcinoma cells Cancer Gene Therapy (2001) doi: 10.1038/sj.cgt.7700305
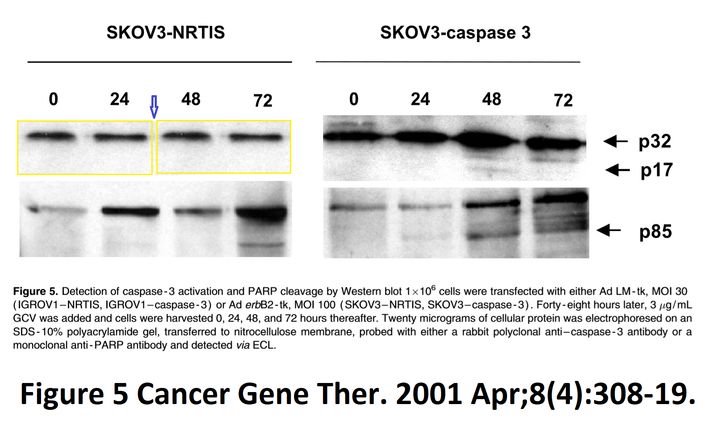
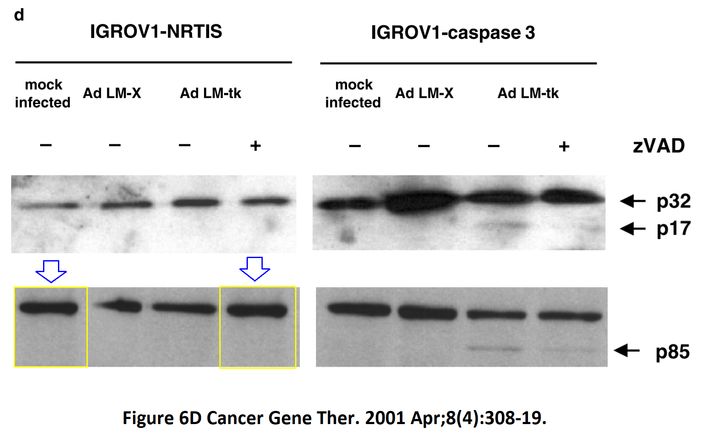
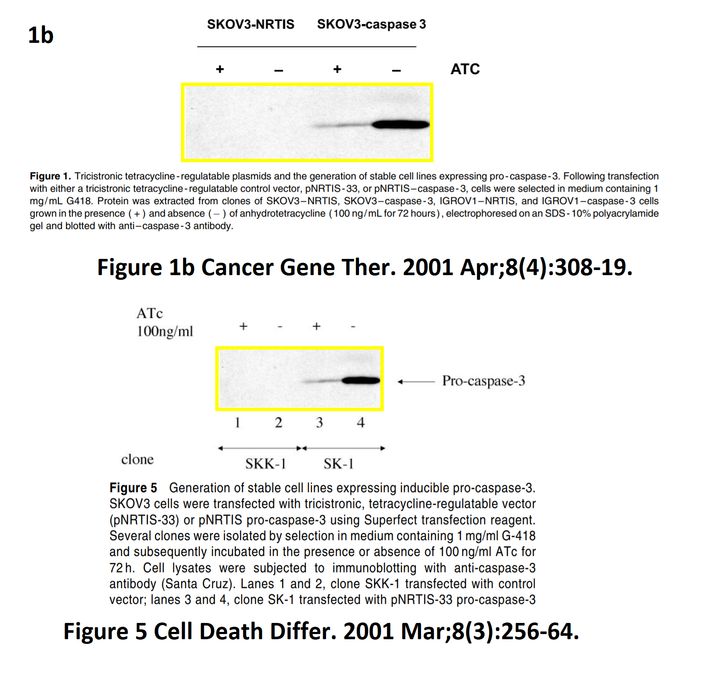
In November 2018, the first author and another Imperial College professor Ian McNeish posted on PubPeer the full investigative report by Limoine’s employer, Barts College, which was also approved by Imperial. McNeish, Limoine and the unnamed investigator agree that the raw data doesn’t exist (anymore, they say), that the two papers were submitted and reviewed simultaneously and that the gel bands are indeed duplicated. The investigator’s conclusions, as quoted by McNeish:
“None of these issues in my opinion change the conclusions made in the papers. I see no merit in retracting these important publications or publishing an erratum at this late stage, although agree that there have been errors made in producing the figures. […] The investigation stated that the conclusions made from the published studies were valid and important, even accounting for these reporting errors.“
This is the standard attitude of all English and in fact British universities. The wording is typical English passive-aggressiveness towards whistleblowers warning them never to waste the university’s time ever again or meet their due fate.
Below some more of Limoine papers, the rest is on PubPeer. The following is 7 years old, when it was posted the raw data was rather fresh, sometimes even piping hot. A key coauthor on all these papers is Yaohe Wang, another professor at Barts.
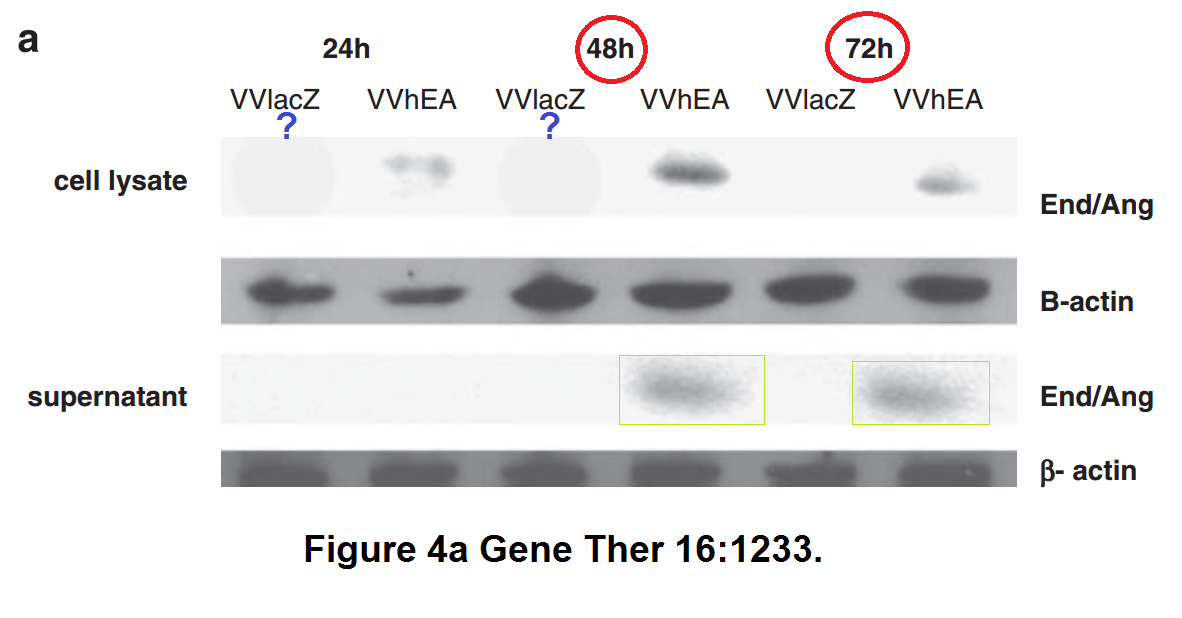
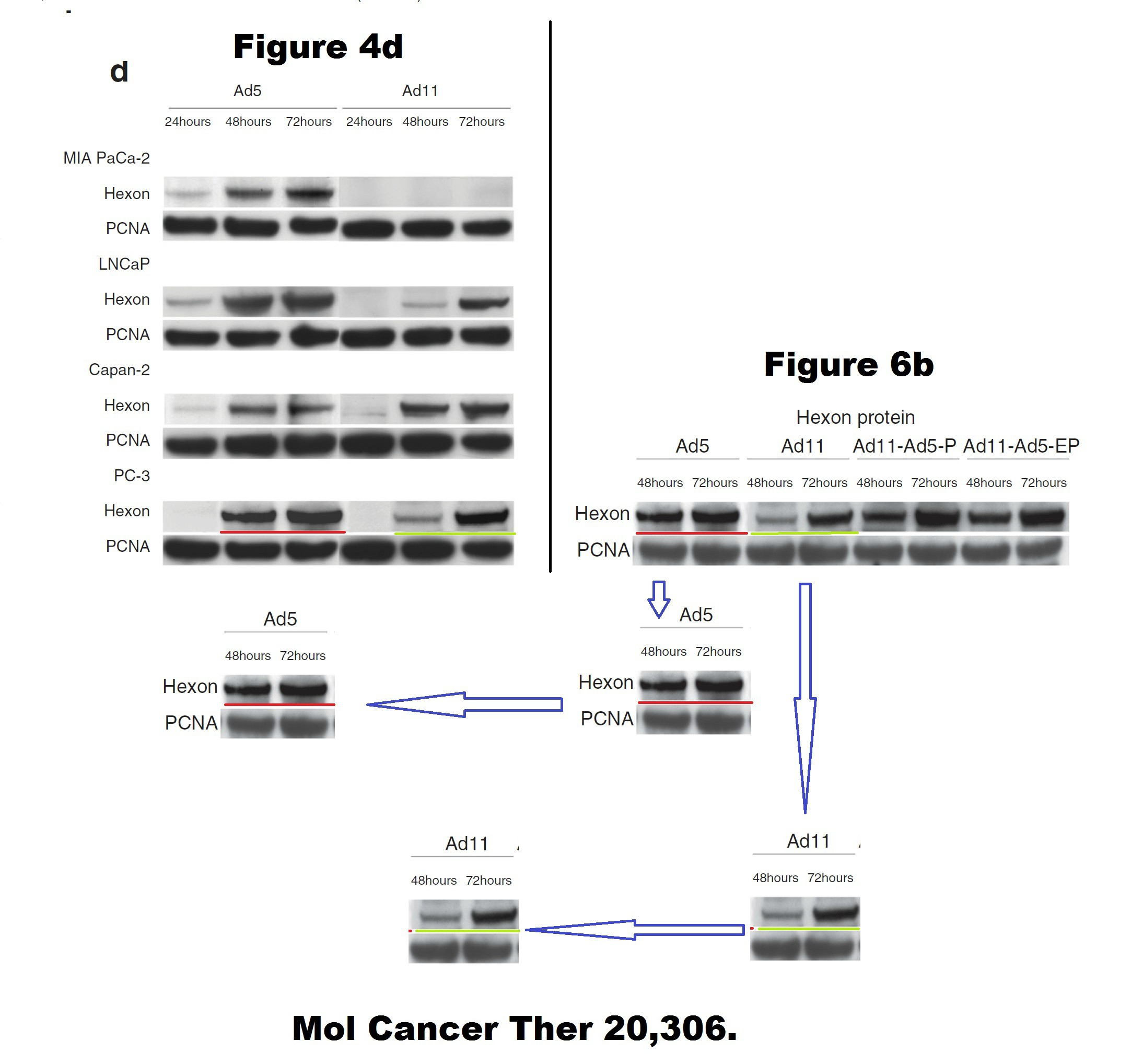
Barts College apparently decided instead to wait until this Photoshop travesty passes the 10 year statute of limitations. Expect another passive-aggressive investigative report in 2025, declaring Limoine and Wang innocent and all conclusions valid and not affected.
Peter St George-Hyslop, OC, FRS, FRSC, FRCPC
I think this name is so impressive, you can’t be more of English upper class, both in science and in society with a name like this. Of course Professor Peter St George-Hyslop is located in Cambridge, where he works on clinical neuroscience, previously he was in Canada where he was proclaimed “one of the most cited authors in the field of Alzheimer’s disease research”.
There are a number of papers coauthored by St George-Hyslop on PubPeer, here is one from his old lab at the University of Toronto:
Fusheng Chen, Gang Yu, Shigeki Arawaka, Masaki Nishimura, Toshitaka Kawarai, Haung Yu, Anurag Tandon, Agnes Supala, You Qiang Song, Ekaterina Rogaeva, Paul Milman, Christine Sato, Cong Yu, Christopher Janus, Julie Lee, Lixin Song, Lili Zhang, Paul E. Fraser, P. H. St George-Hyslop Nicastrin binds to membrane-tethered Notch Nature Cell Biology (2001) doi: 10.1038/35087069
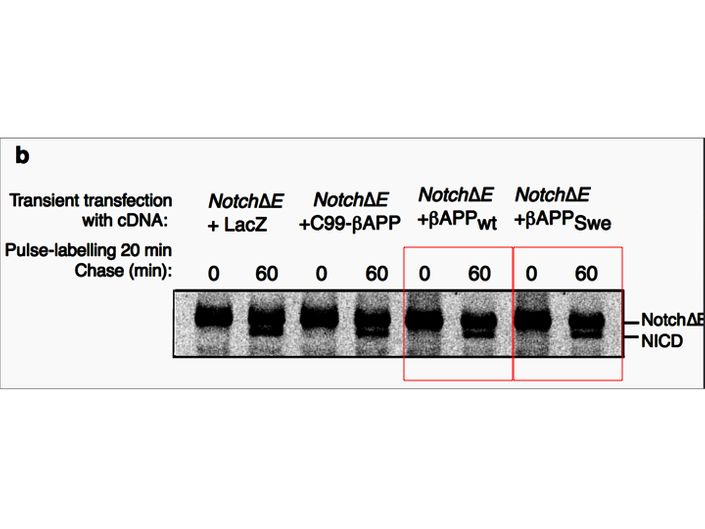
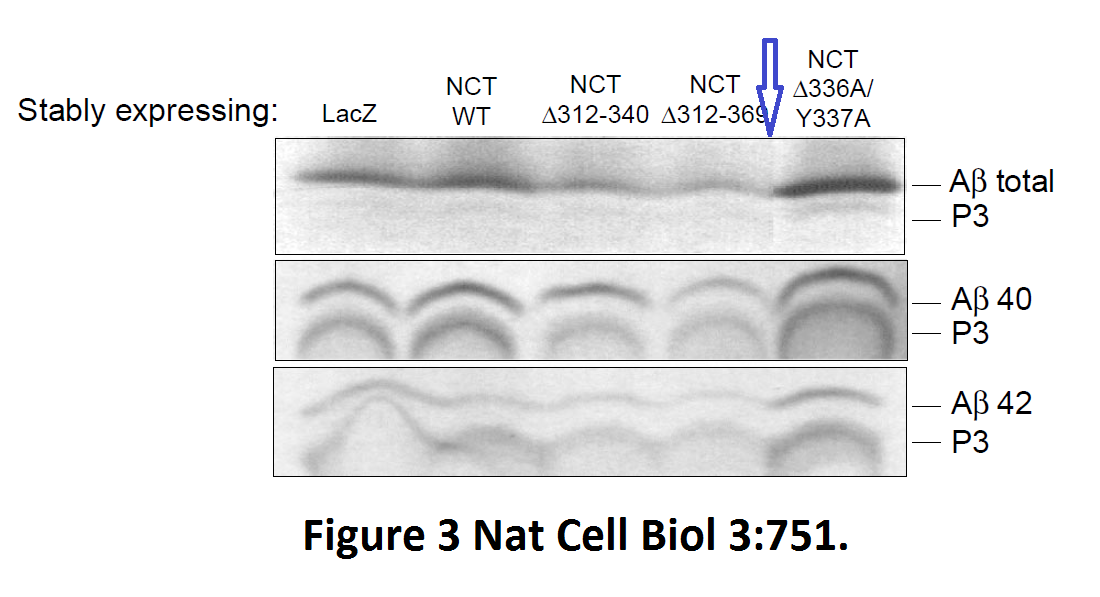
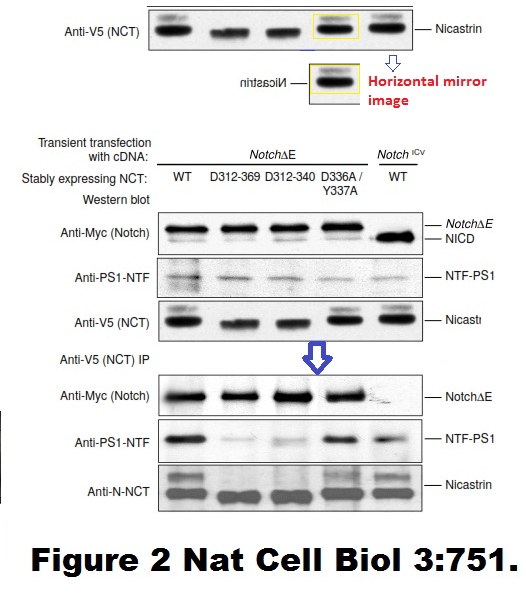
Cloned gel bands, spotted by Clare Francis. No action at all form that elite Nature-themed journal. Another St George-Hyslop fabrication, also done with Toronto colleagues:
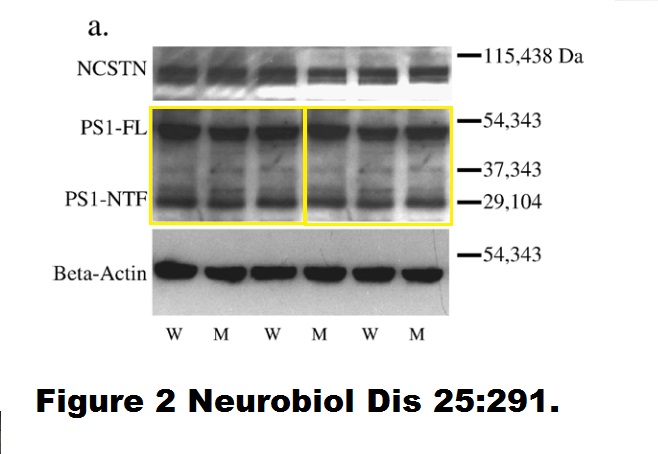
Sonya Brijbassi, Zareen Amtul, Susan Newbigging, David Westaway, Peter St George-Hyslop, Richard F. Rozmahel Excess of nicastrin in brain results in heterozygosity having no effect on endogenous APP processing and amyloid peptide levels in vivo Neurobiology of Disease (2007) doi: 10.1016/j.nbd.2006.09.013
But the worst ones were done in collaboration with Frederic Checler, professor of neuroscience at CNRS in France. Like this, where the authors shamelessly double-published to increase their publication record:
Julie Dunys, Toshitaka Kawarai, Emilie Giaime, Sherwin Wilk, M. Herrant, P. Auberger, Peter St George-Hyslop, Cristine Alves Da Costa, Frédéric Checler Study on the putative contribution of caspases and the proteasome to the degradation of Aph-1a and Pen-2 Neuro-degenerative diseases (2007) doi: 10.1159/000101840
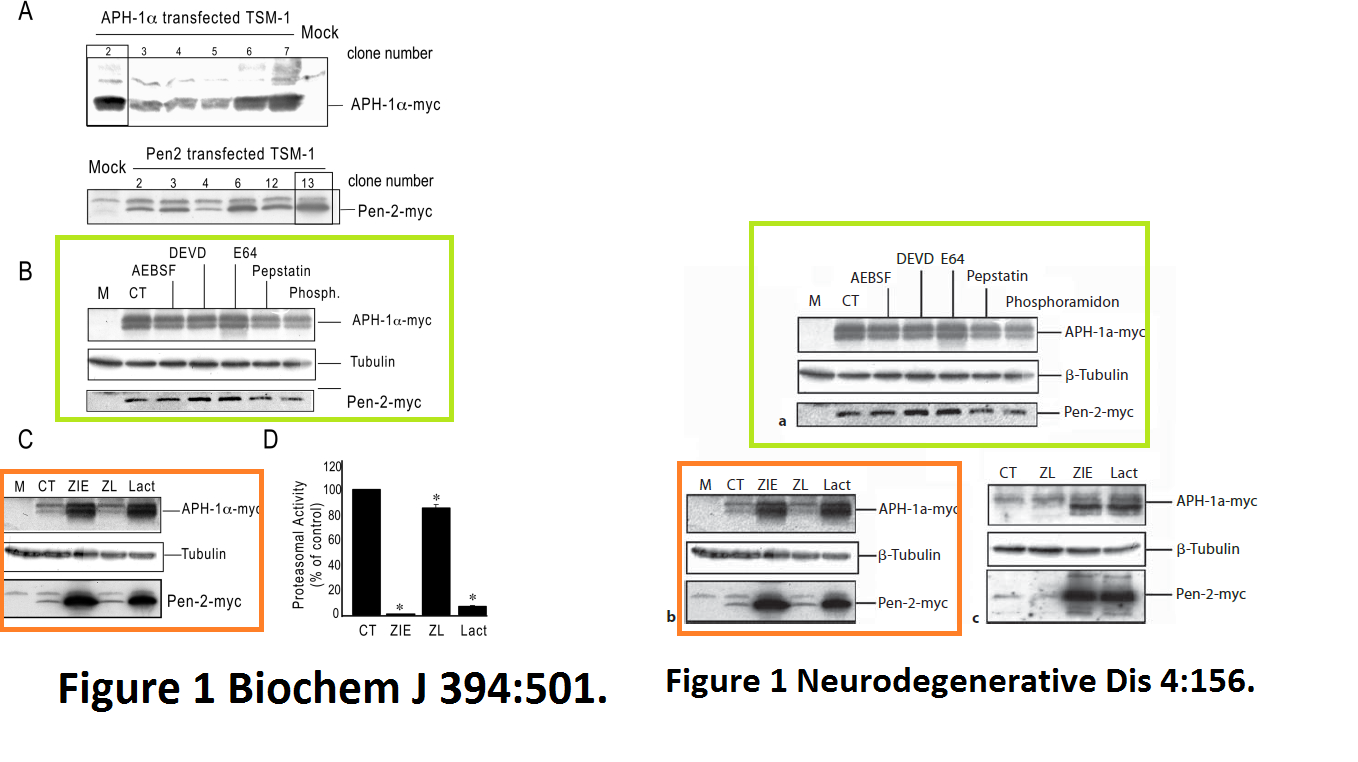
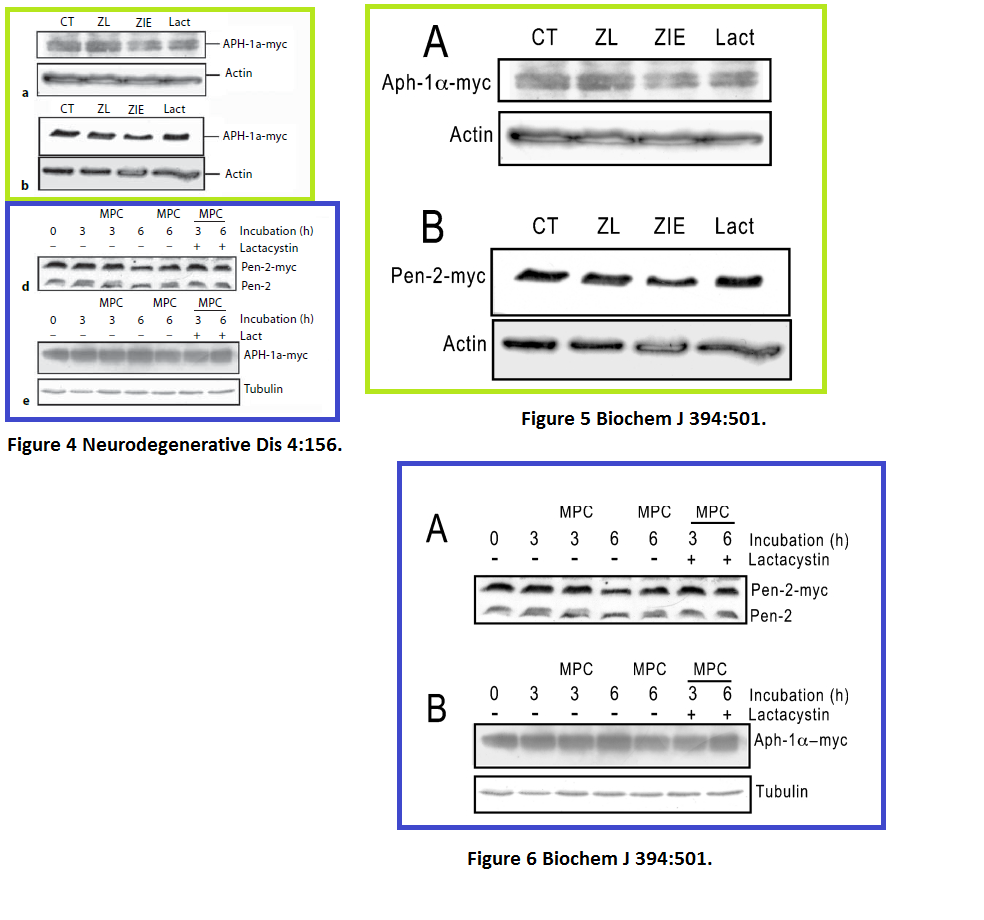
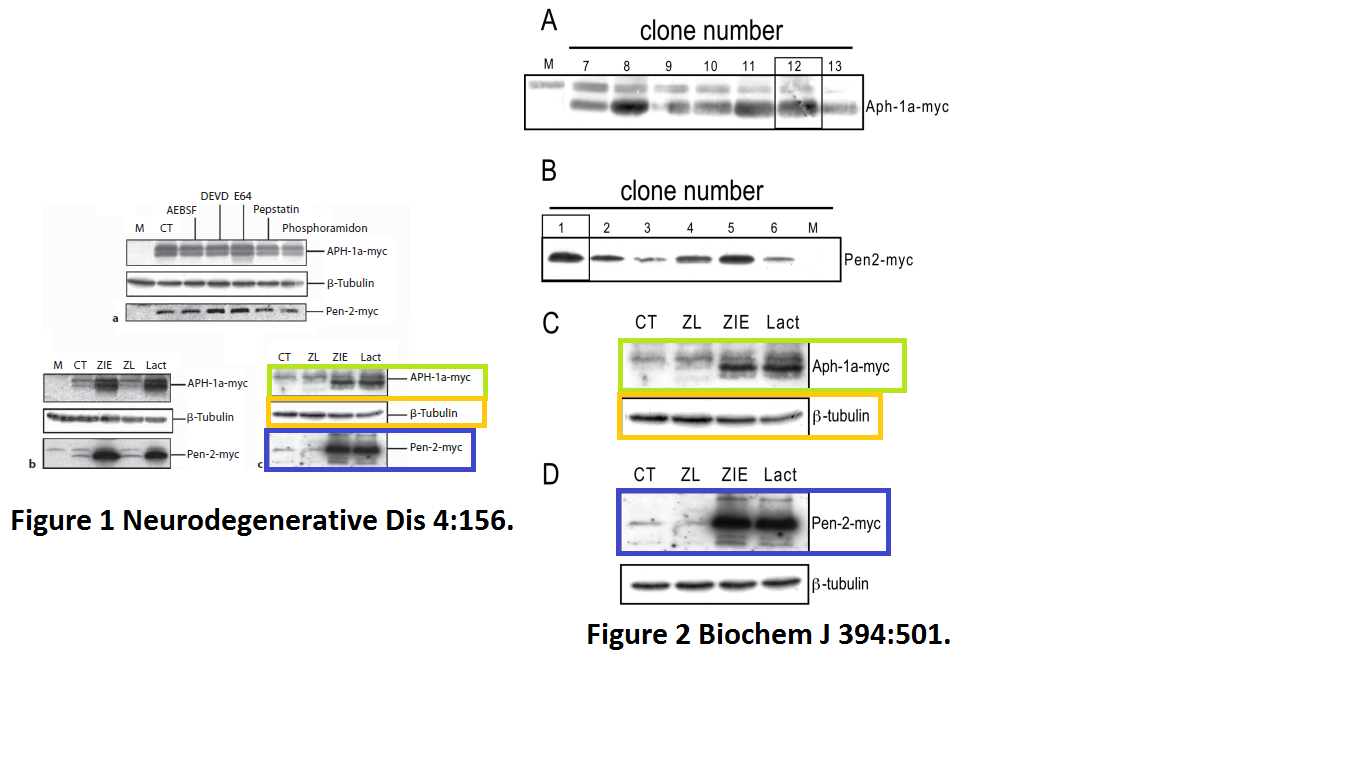
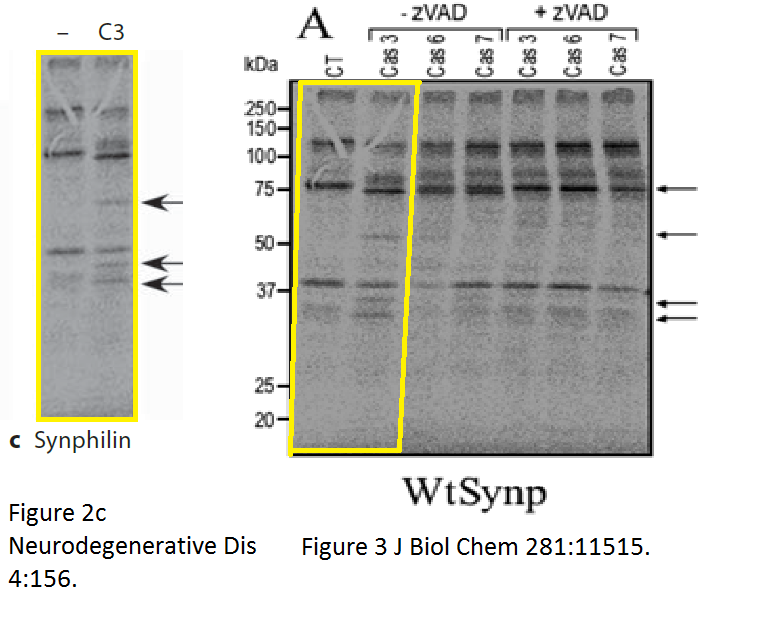
There was more data recycling, basically that paper in Neurodeg Dis is a copy of the previously published paper Dunys et al Biochemical J 2006, with all other authors the same. Neither journal, one by Portland Press, one by Karger, cares, never mind for research integrity, not even for their own infringed copyright.
Here more by St George-Hylop and his French ally, some gel band copy-pasting:
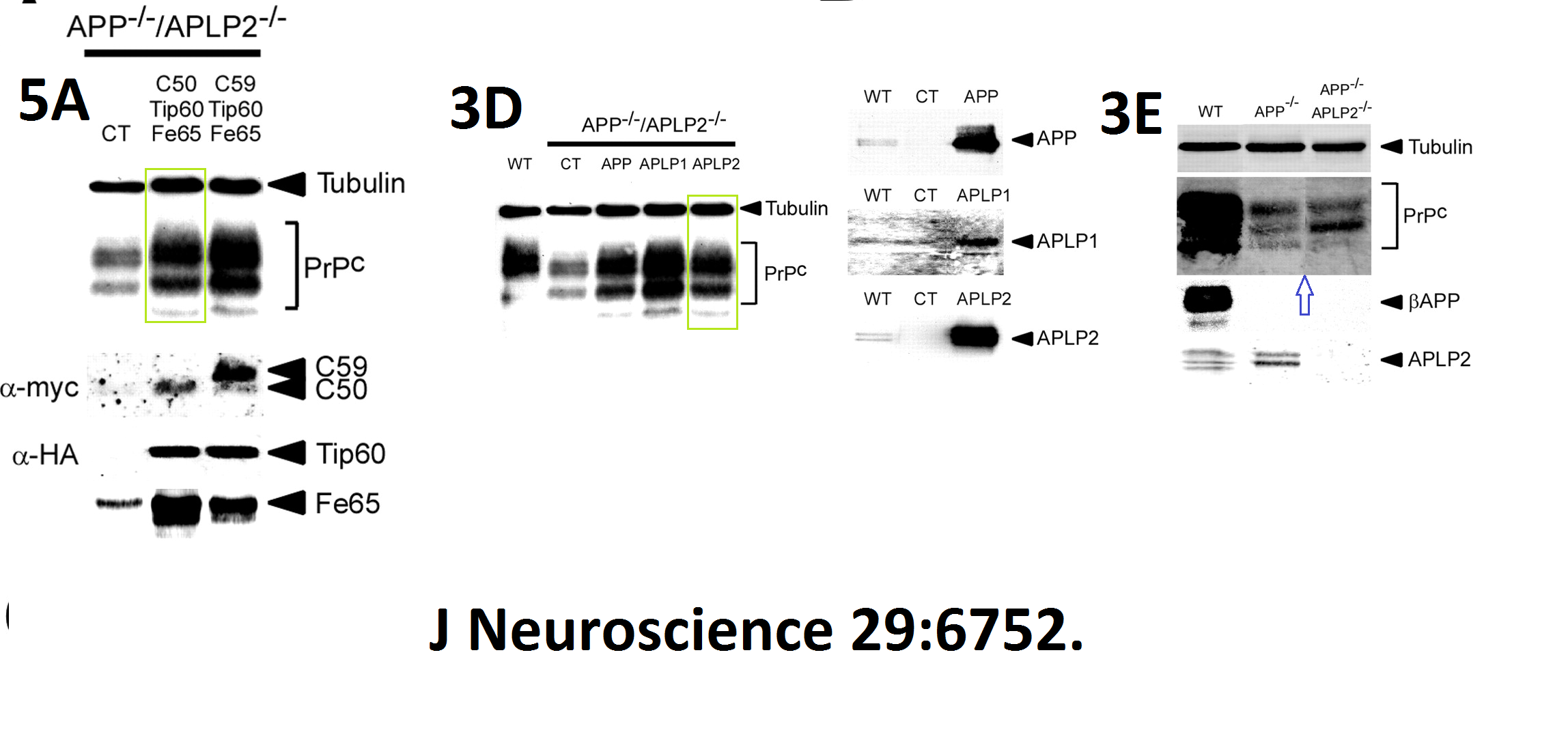
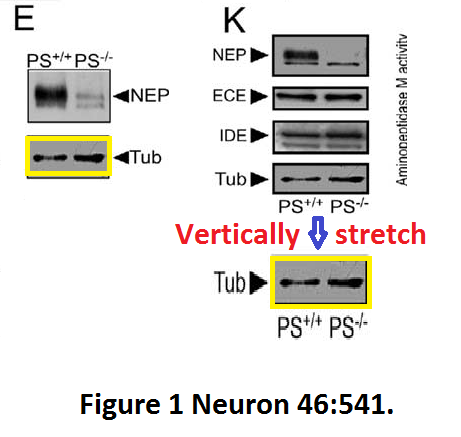
Here another interesting collaboration of our esteemed English upper class scientist, this time with Masaya Tohyama in Japan:
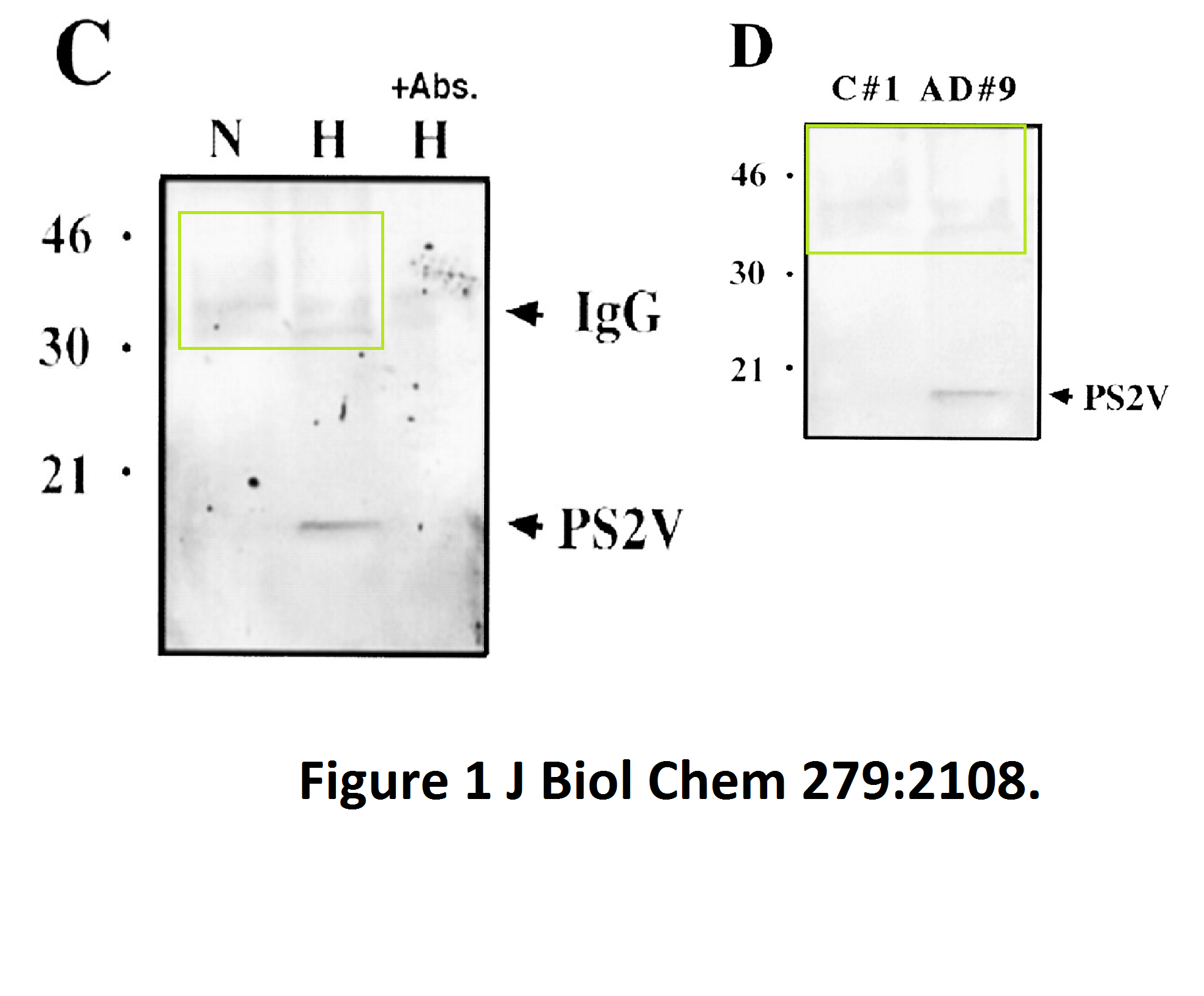
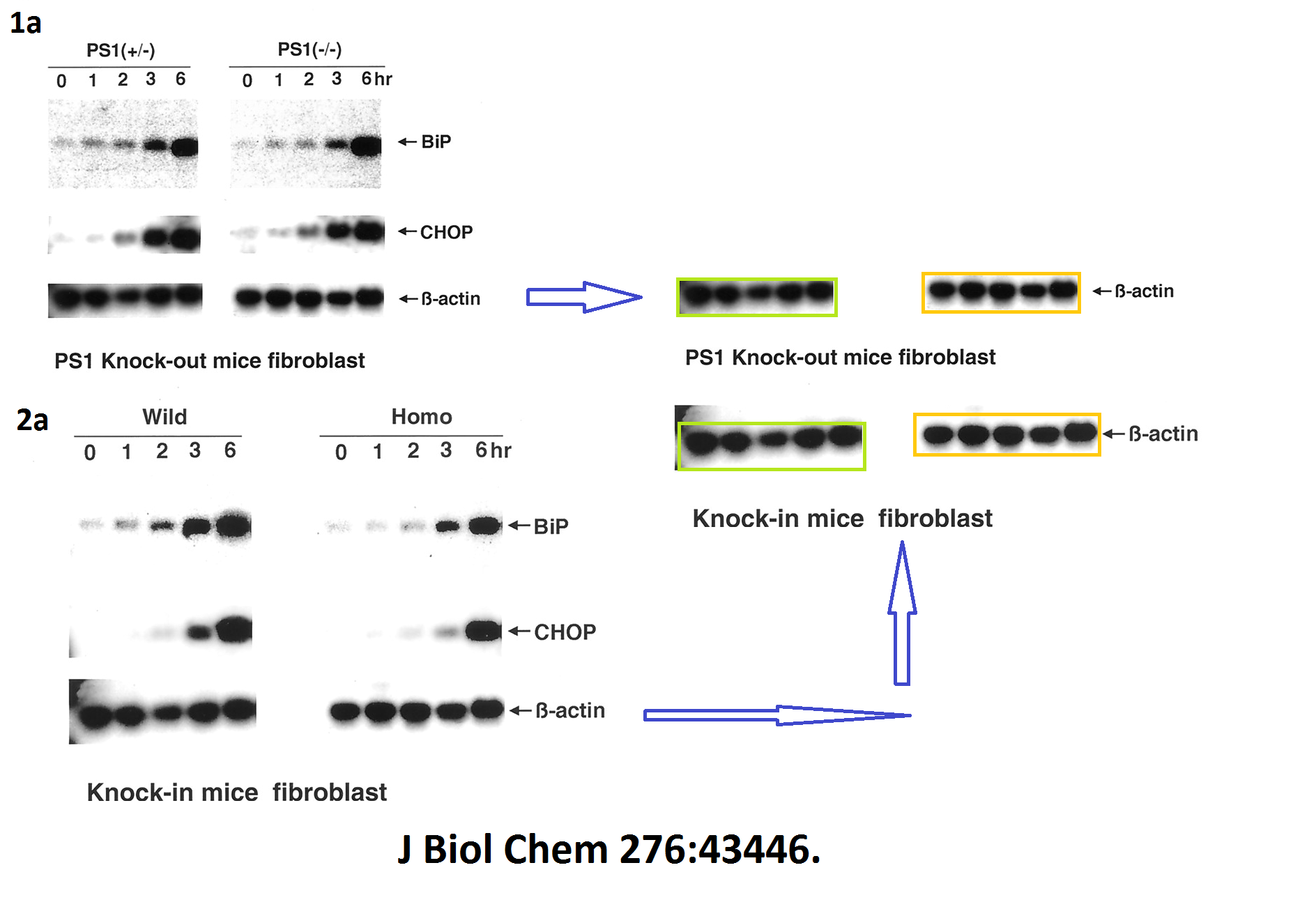
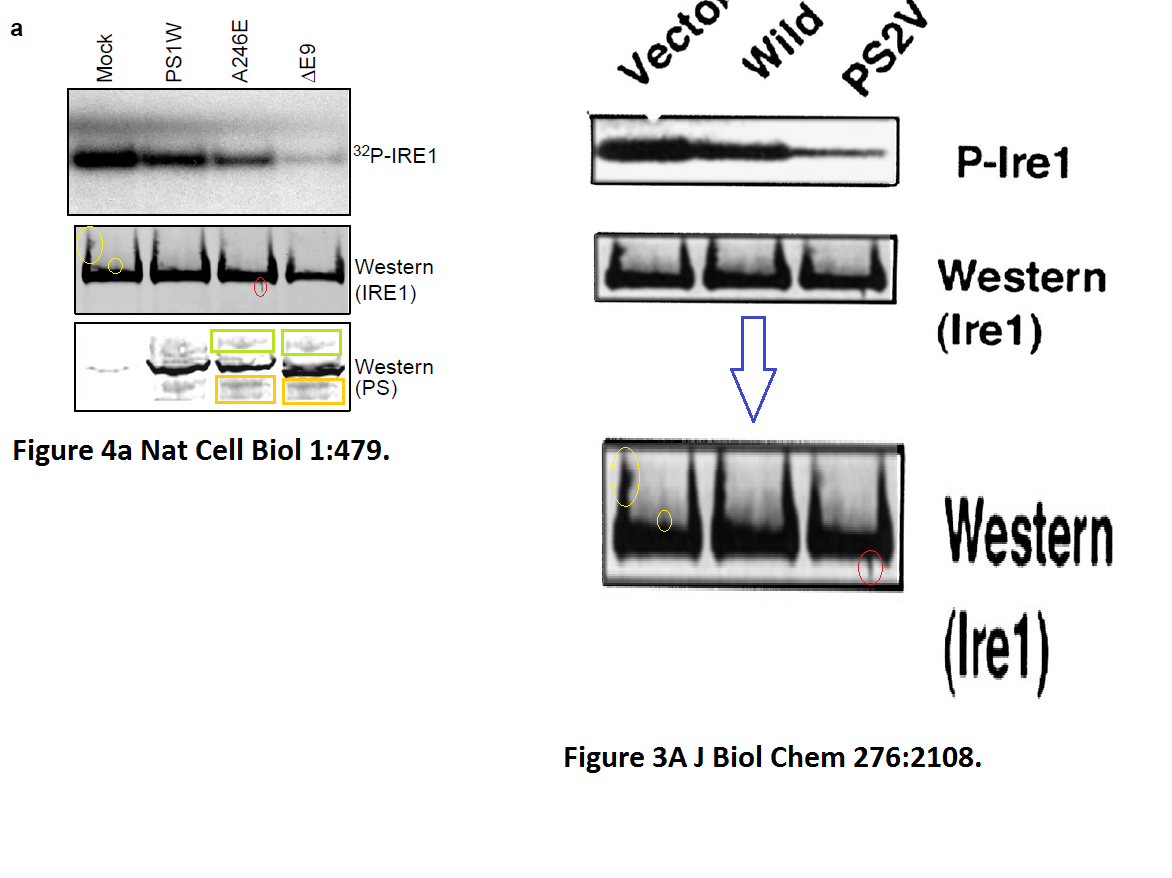
The evidence is years old, but nothing has happened so far. Yet one St George-Hyslop coauthored publication was retracted in 2020. Its first author Sabine Wislet is professor in Belgium and has a number of other papers questioned on PubPeer, including another retraction.
Sabine Wislet-Gendebien, Cheryl D’Souza, Toshitaka Kawarai, Peter St George-Hyslop, David Westaway, Paul Fraser, Anurag Tandon Cytosolic proteins regulate alpha-synuclein dissociation from presynaptic membranes The Journal of biological chemistry (2006) doi: 10.1074/jbc.m605965200
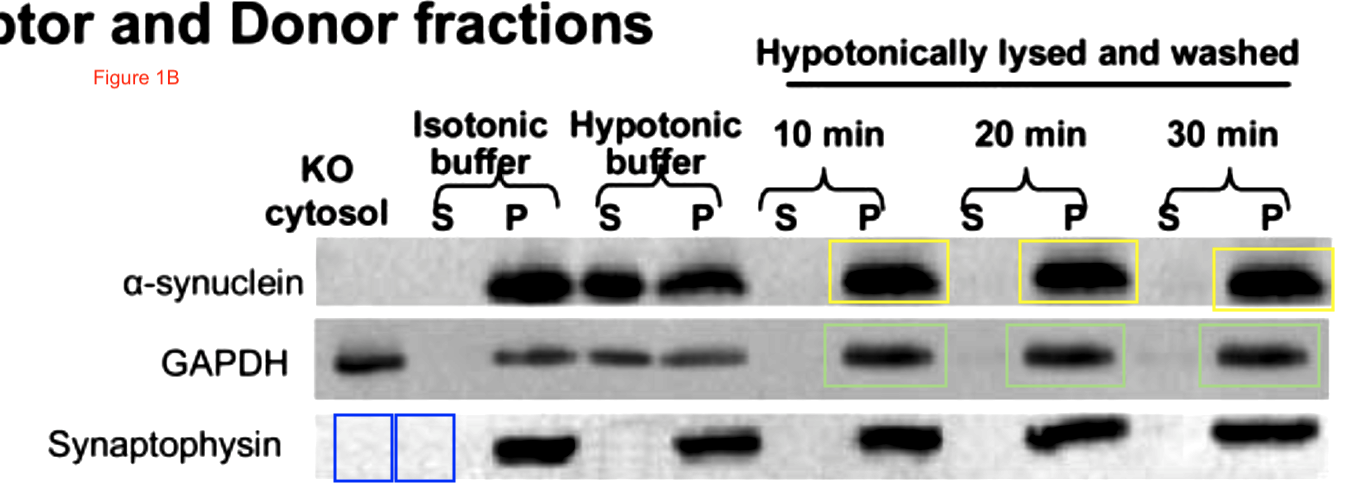
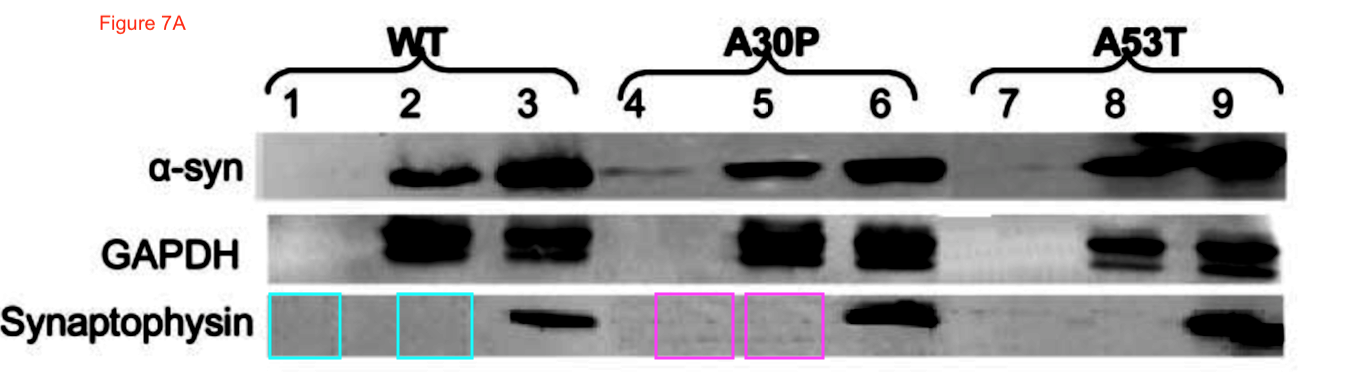
Now the fun starts. Clare Francis of course notified the University of Cambridge and the Wellcome Trust (which funds St Seorge-Hyslop’s research). This was their decision, as penned to Clare Francis by the Policies & Governance Officer of Wellcome Trust, Lucy Douch, in February 2017:
“Further to your email of 17 August 2016, we raised your concerns about Professor Peter St George-Hyslop with the University of Cambridge, the employing institution.
The University conducted an investigation under its Misconduct in Research policy. The investigation concluded that the allegations made are entirely unfounded.”
This is not even passive aggressive, it sounds almost like a legal threat from Wellcome Trust. In fact, this is a proud English Fuck You to every single whistleblower who dares to impugn the greatness of English science genius.
Although, to be fair, I suspect the Cambridge University probably has same statute of limitations on research fraud as Oxford, of 3 years. Because there was nothing for them to investigate in those older paper, they declared the allegations as “entirely unfounded”.
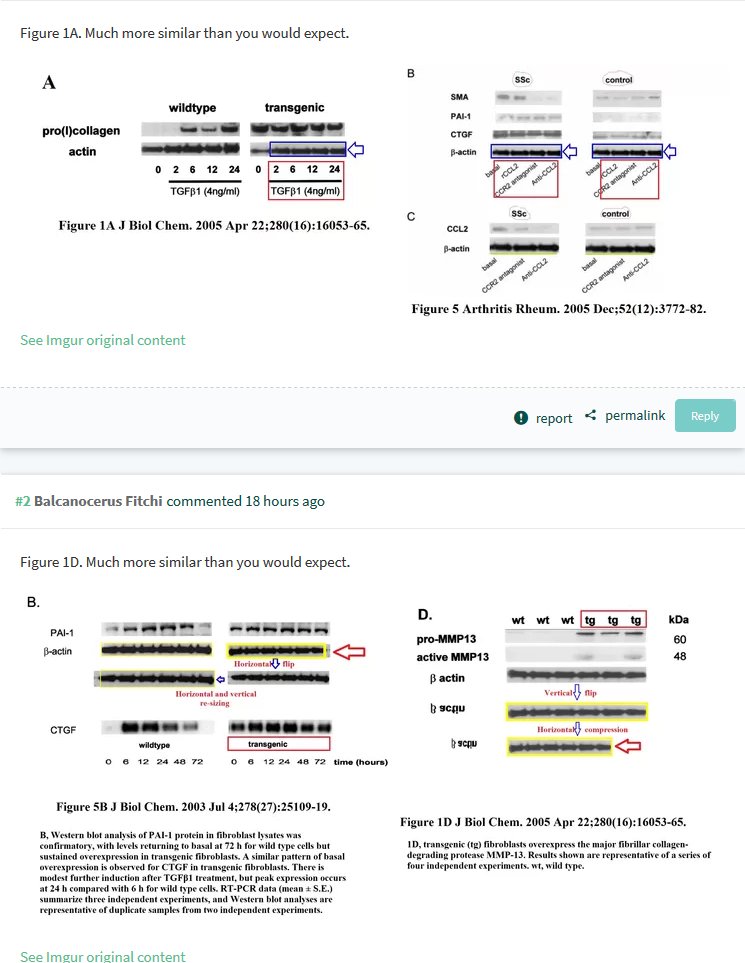
If you enjoyed St George-Hyslop’s aristocratic antics, you should also have a look at the PubPeer record of another Cambridge professor, Dame Carol Mary Black, DBE, FRCP, BSD, Principal of the Newnham College. Here a sample, with the Dame Commander of the Order of the British Empire as last author, Denton et al JBC 2005:
Xin Lu, FRS
Britain is open to foreigners, as we know. Not the lowly kind who don’t know how to get rich quick. If you know how to play the game, you can become part of the English science elite. Xin Lu studied in her home land China, and in UK she eventually arrived to the Ludwig Cancer Centre, first at its London branch and later to take over the directorship of the central seat in Oxford.
Yes, Oxford is the university with 3 year statute of limitation for research misconduct. Nothing to see here at all:
Daniele Bergamaschi, Yardena Samuels, Alexandra Sullivan, Marketa Zvelebil, Hilde Breyssens, Andrea Bisso, Giannino Del Sal, Nelofer Syed, Paul Smith, Milena Gasco, Tim Crook, Xin Lu iASPP preferentially binds p53 proline-rich region and modulates apoptotic function of codon 72–polymorphic p53 Nature Genetics (2006) doi: 10.1038/ng1879
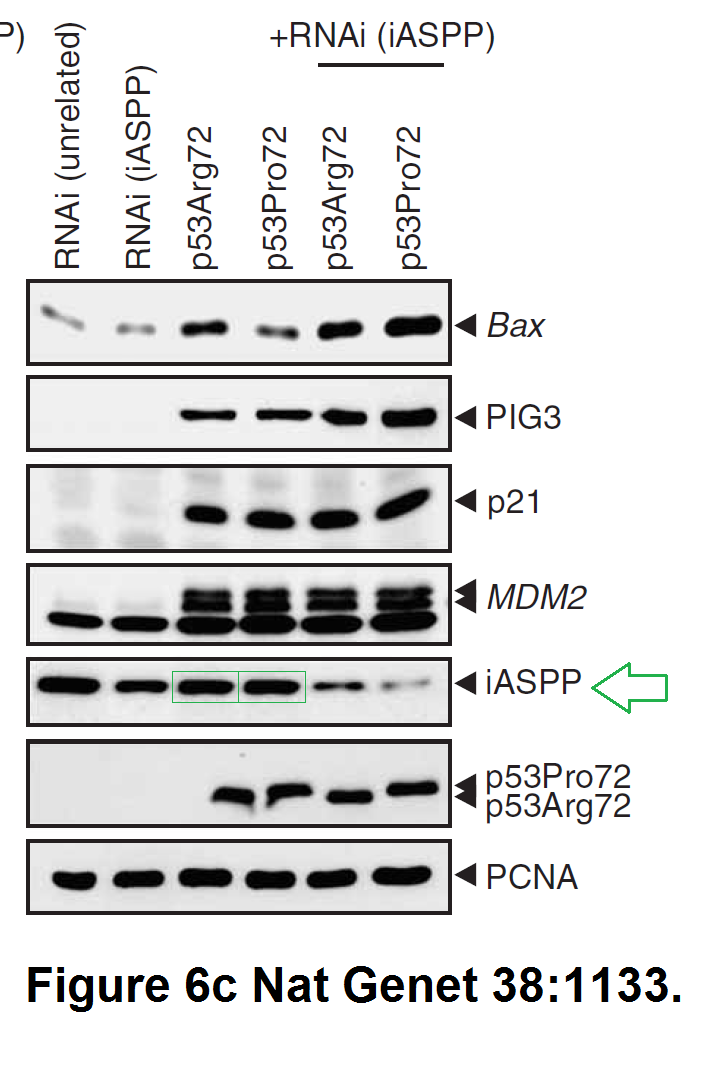
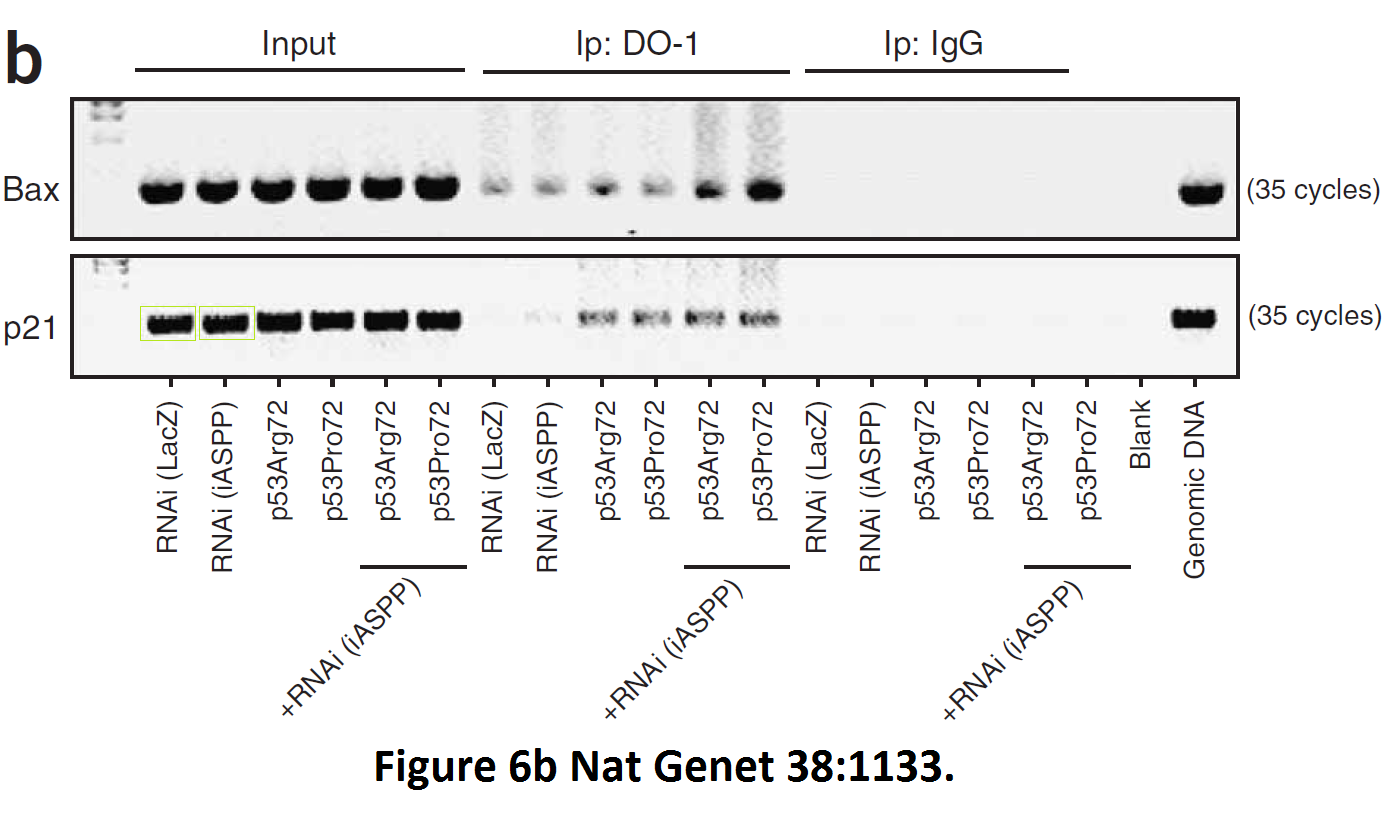
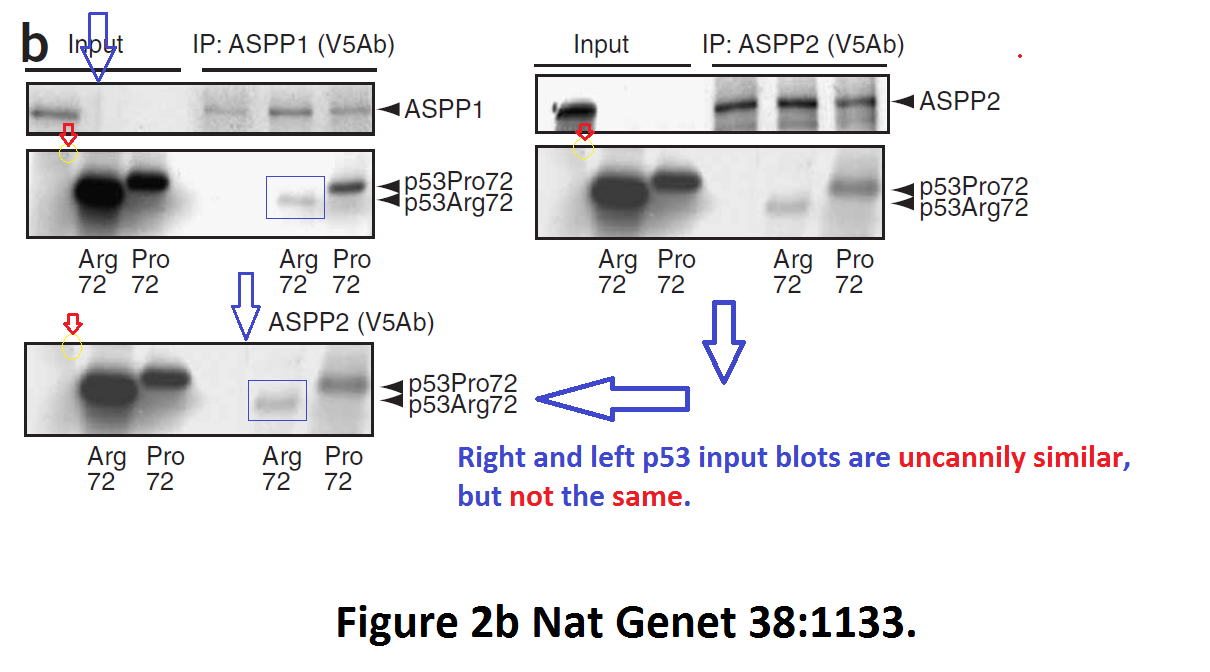
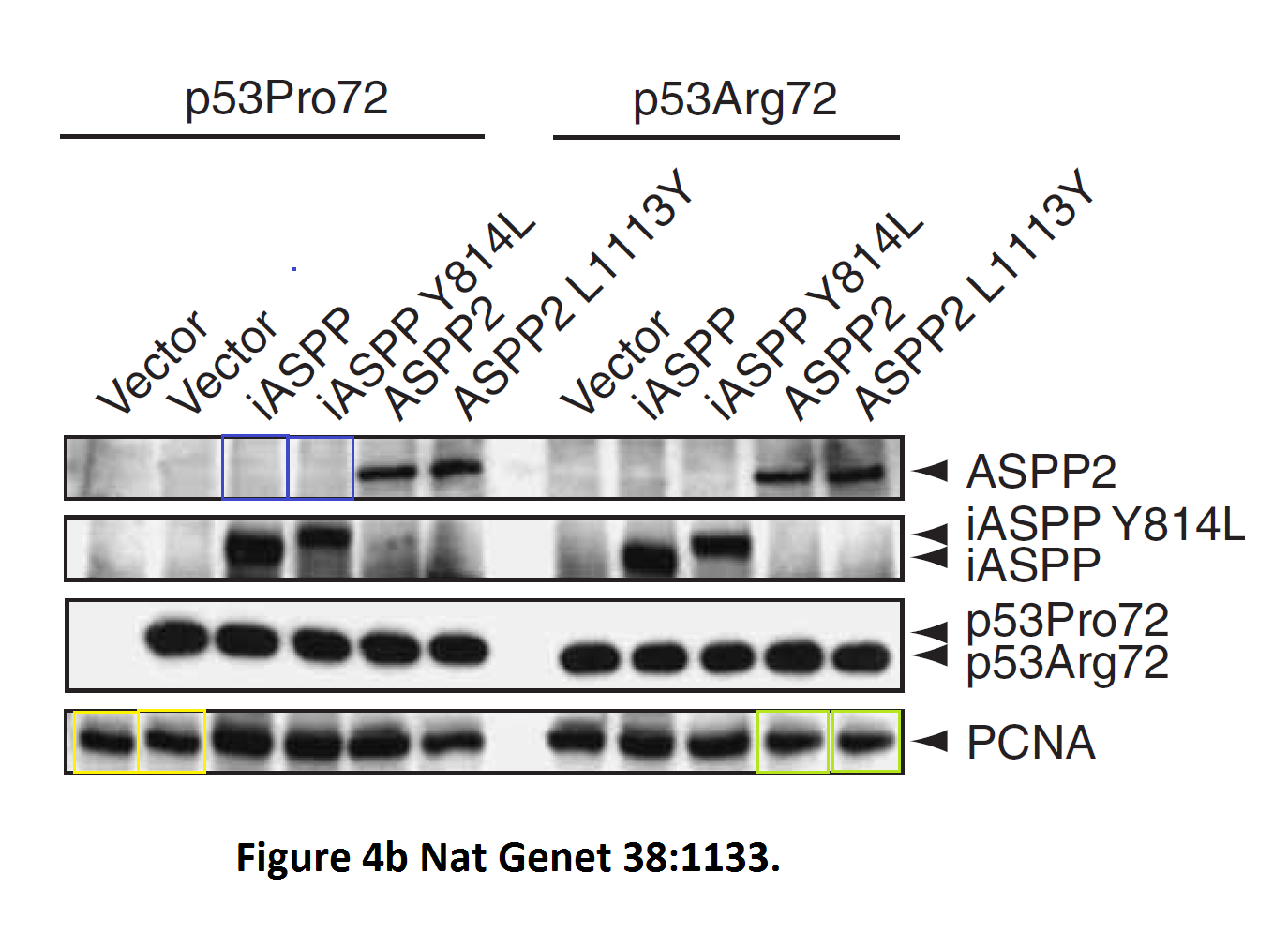
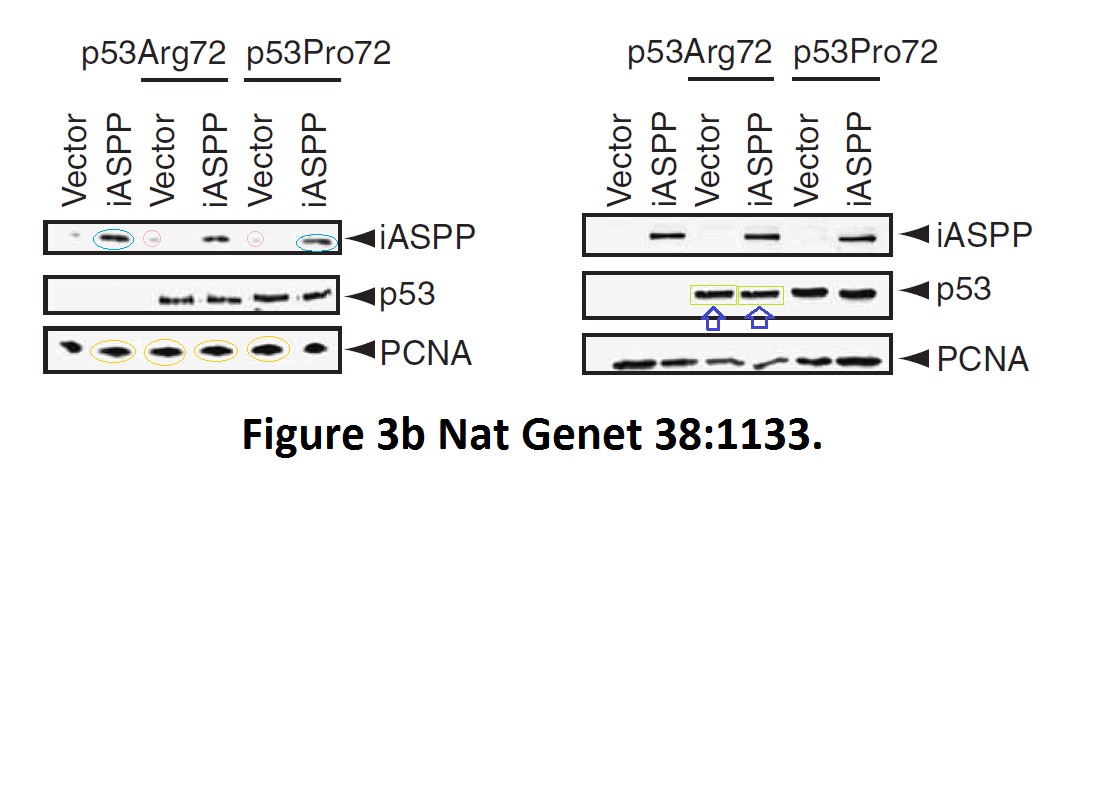
So many cloned gel bands, and yes, Clare Francis informed the University of Oxford of this and other masterpieces. The first author on that paper, Daniele Bergamaschi is now lecturer at the Queen Mary University of London, his lab is at Barts. You see again which kind of talents Barts boss Lemoine expects from his principal investigators. Bergamaschi also authored this work of art with Lu:
Daniele Bergamaschi, Yardena Samuels, Boquan Jin, Sai Duraisingham, Tim Crook, Xin Lu ASPP1 and ASPP2: common activators of p53 family members Molecular and Cellular Biology (2004) doi: 10.1128/mcb.24.3.1341-1350.2004
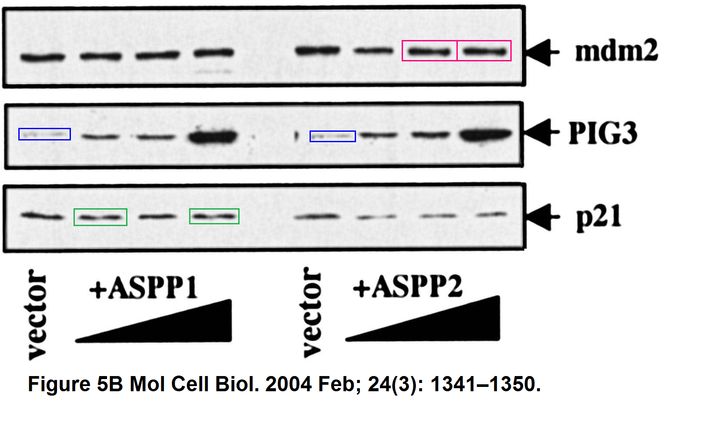
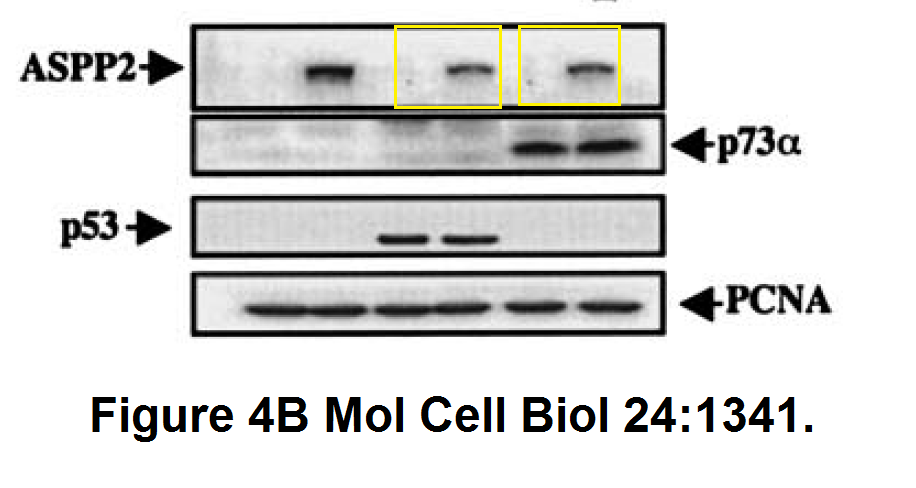
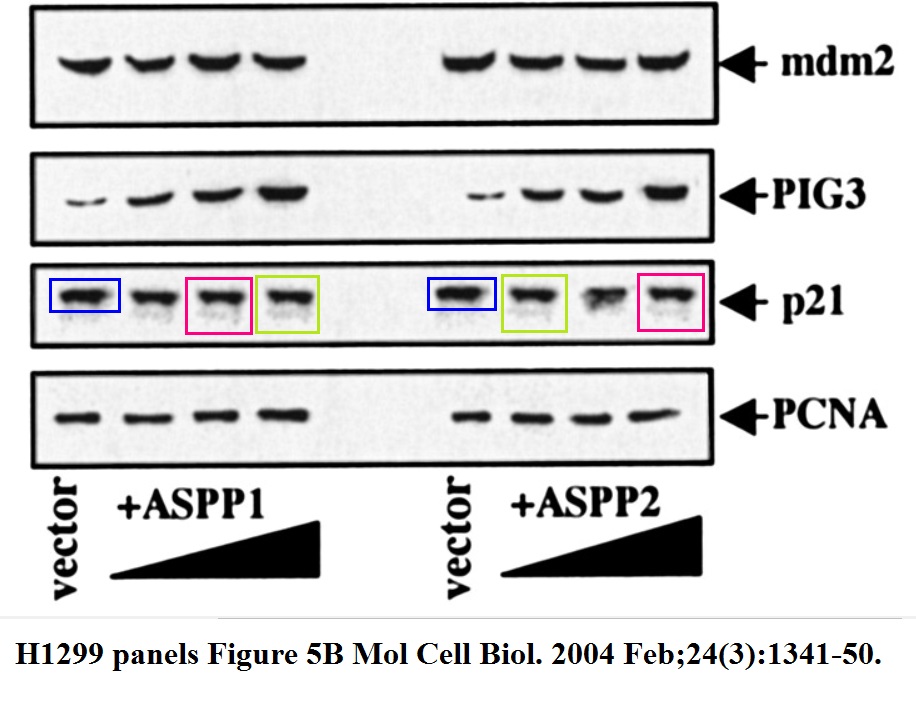
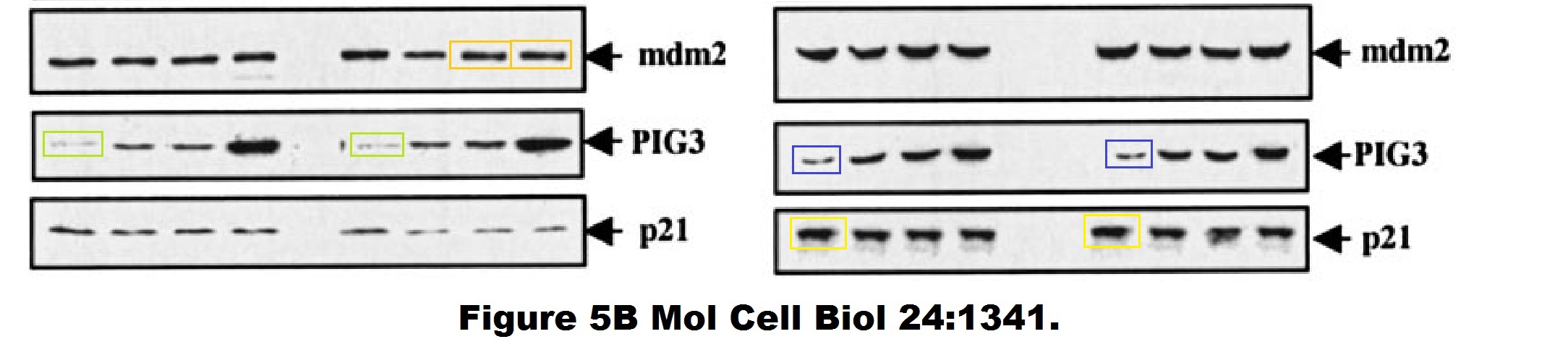
And this one by Bergamaschi and Lu, in Oncogene (remember the Editor-in-Chief Justin Stebbing?):
Daniele Bergamaschi, Yardena Samuels, Shan Zhong, Xin Lu Mdm2 and mdmX prevent ASPP1 and ASPP2 from stimulating p53 without targeting p53 for degradation Oncogene (2005) doi: 10.1038/sj.onc.1208535
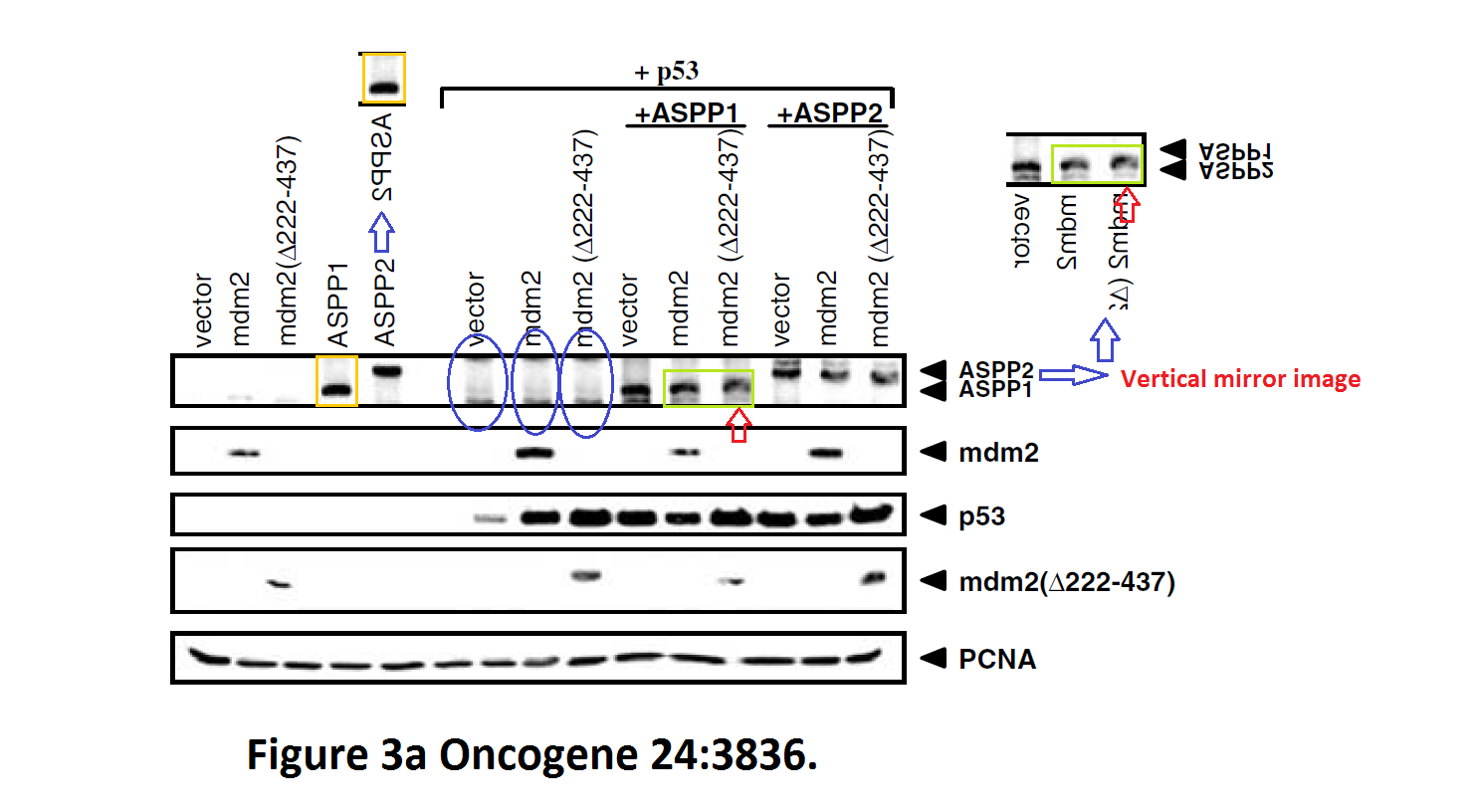
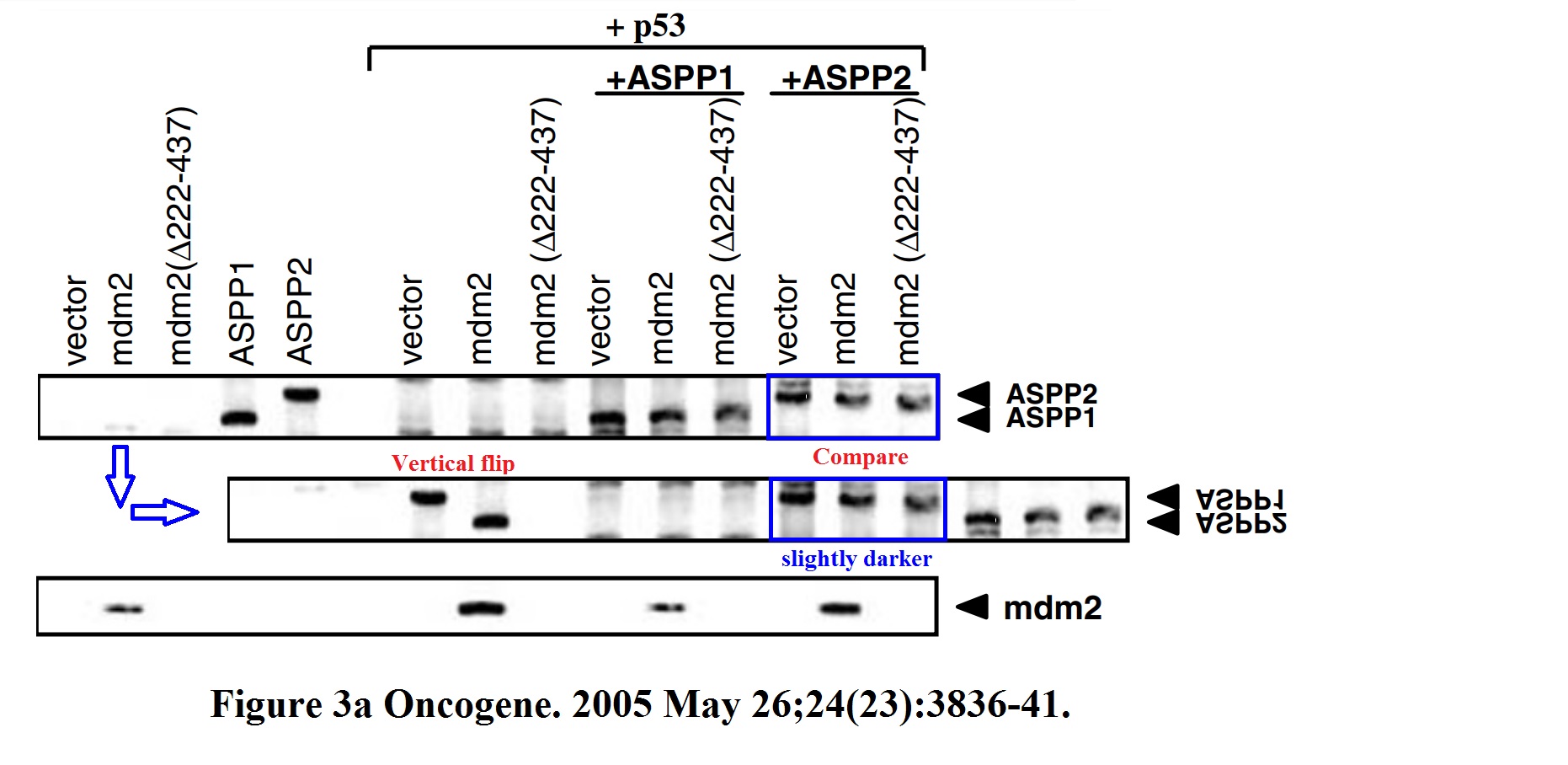
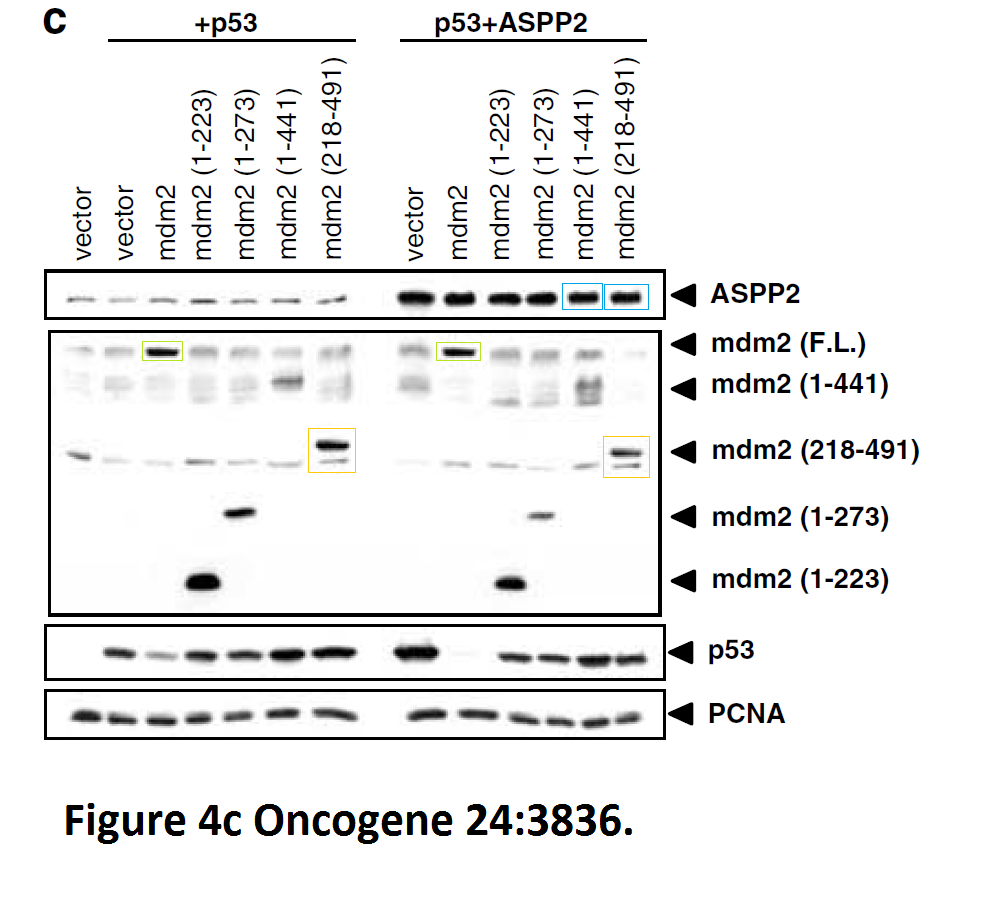
I hope you are learning from Bergamaschi and Lu how to make career in English science. It’s not that difficult, all you need is the will to succeed. Another common author on all these papers is Yardena Samuels. She went back to Israel where she is now professor at the Weizmann Institute. Here more by Bergamaschi (and Samuels):
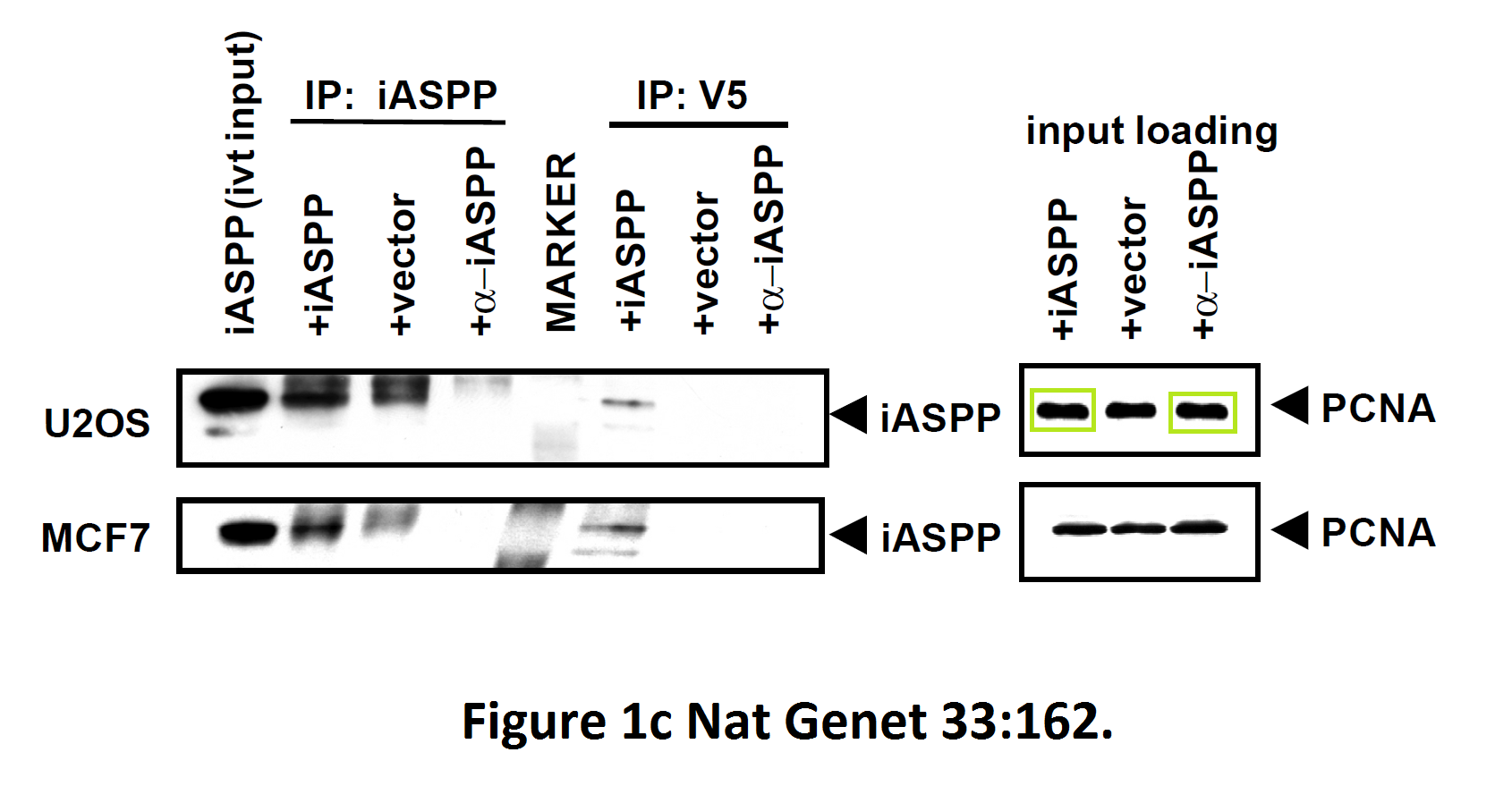
The last author on one of those papers is Geoffrey Smith, head of Pathology Department at University of Cambridge and member of all possible learned societies including the German Leopoldina. People of this calibre can’t be associated with bad science, so don’t expect even a correction.
But the next two papers from Lu lab doesn’t have Bergamaschi among its authors. Here one:

Damian B. S. Yap, Jung-Kuang Hsieh, Shan Zhong, Vicky Heath, Barry Gusterson, Tim Crook, Xin Lu Ser392 phosphorylation regulates the oncogenic function of mutant p53 Cancer Research (2004) doi: 10.1158/0008-5472.can-1305-2
These are two very fraudulent figures, bands were erased in one version by a rascal. But this paper, like others, is too old for anyone to give a toss about. Here something more recent:
Min Lu, Hilde Breyssens, Victoria Salter, Shan Zhong, Ying Hu, Caroline Baer, Indrika Ratnayaka, Alex Sullivan, Nicholas R. Brown, Jane Endicott, Stefan Knapp, Benedikt M. Kessler, Mark R. Middleton, Christian Siebold, E. Yvonne Jones, Elena V. Sviderskaya, Jonathan Cebon, Thomas John, Otavia L. Caballero, Colin R. Goding, Xin Lu Restoring p53 function in human melanoma cells by inhibiting MDM2 and cyclin B1/CDK1-phosphorylated nuclear iASPP Cancer Cell (2013) doi: 10.1016/j.ccr.2013.03.013
An Erratum was issued by Cell Press in 2016 and declared:
“This error does not affect any of the findings reported in the paper.”
With English research fabrications it is just like with Brexit: get over it, they won. Science is an English colony and the rotten upper classes are busy plundering it.
Donate!
If you are interested to support my work, you can leave here a small tip of $5. Or several of small tips, just increase the amount as you like (2x=€10; 5x=€25). Or you can donate to Barts or Ludwig centres for cancer research!
€5.00


Genes Dev. 2009 May 15; 23(10): 1177–1182.doi: 10.1101/gad.511109PMCID: PMC2685533PMID: 19451218
Histone demethylase JMJD3 contributes to epigenetic control of INK4a/ARF by oncogenic RAS
Marta Barradas,1,6 Emma Anderton,2,6 Juan Carlos Acosta,1,6 SiDe Li,3,4 Ana Banito,1 Marc Rodriguez-Niedenführ,2 Goedele Maertens,2 Michaela Banck,5 Ming-Ming Zhou,4 Martin J. Walsh,3,4 Gordon Peters,2,8 and Jesús Gil1,7
1Cell Proliferation Group, MRC Clinical Sciences Centre, Imperial College, London W12 0NN, United Kingdom;
2Molecular Oncology Laboratory, CRUK London Research Institute, London WC2A 3PX, United Kingdom;
3Department of Pediatrics, Mount Sinai School of Medicine, New York, New York 10029, USA;
4Department of Structural and Chemical Biology, Mount Sinai School of Medicine, New York, New York 10029, USA;51Cell Proliferation Group, MRC Clinical Sciences Centre, Imperial College, London W12 0NN, United Kingdom;
5Department of Medicine, Division of Hematology and Oncology, Mount Sinai School of Medicine, New York, New York 10029, USA
5Department of Medicine, Division of Hematology and Oncology, Mount Sinai School of Medicine, New York, New York 10029, USA
6These authors contributed equally to this work.
7Corresponding authors.E-MAIL ku.ca.crm.csc@lig.susej; FAX 44-20-8383-8306.
8E-MAIL ku.gro.recnac@sretep.nodrog; FAX 44-207-269-3094.
Problematic data figure 3C.
p16 panel does not look like it comes from the same blot as CDK4 panel, the stated loading control.
LikeLike
Nat Cell Biol. 2004 Jan;6(1):67-72. doi: 10.1038/ncb1077. Epub 2003 Nov 30.
Polycomb CBX7 has a unifying role in cellular lifespan
Jesús Gil 1, David Bernard, Dolores Martínez, David Beach
Affiliations collapse
Affiliation
1Wolfson Institute for Biomedical Research, University College London, Gower Street, London WC1E 6BT, UK. jesus.gil@cancer.org.uk
PMID: 14647293 DOI: 10.1038/ncb1077
Figure 3c. Much more similar than expected.
https://pubpeer.com/publications/501C39F3EA1E683123948B829365D8
LikeLike
Cell Stem Cell. 2012 Jan 6;10(1):33-46. doi: 10.1016/j.stem.2011.12.004.
MicroRNA regulation of Cbx7 mediates a switch of Polycomb orthologs during ESC differentiation
Ana O’Loghlen 1, Ana M Muñoz-Cabello, Alexandre Gaspar-Maia, Hsan-Au Wu, Ana Banito, Natalia Kunowska, Tomas Racek, Helen N Pemberton, Patrizia Beolchi, Fabrice Lavial, Osamu Masui, Michiel Vermeulen, Thomas Carroll, Johannes Graumann, Edith Heard, Niall Dillon, Veronique Azuara, Ambrosius P Snijders, Gordon Peters, Emily Bernstein, Jesus Gil
Affiliation1Cell Proliferation Group, MRC Clinical Sciences Centre, Imperial College London, Hammersmith Campus, London W12 0NN, UK.PMID: 22226354 PMCID: PMC3277884 DOI: 10.1016/j.stem.2011.12.004
Problematic data figure 1. Much more similar than expected.
LikeLike
Gut. 2007 Jan;56(1):95-106. doi: 10.1136/gut.2005.083691.https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1856675/
Pancreatic cancer cells overexpress gelsolin family‐capping proteins, which contribute to their cell motility
C C Thompson, F J Ashcroft, S Patel, G Saraga, D Vimalachandran, E Costello, Division of Surgery and Oncology, Royal Liverpool University Hospital, University of Liverpool, Liverpool, UK
W Prime, Cancer Tissue Bank Research Centre, Department of Pathology, University of Liverpool, Liverpool, UK
F Campbell, A Dodson, Department of Pathology, University of Liverpool, Liverpool, UK
R E Jenkins, Department of Pharmacology and Therapeutics, University of Liverpool, Liverpool, UK
N R Lemoine, T Crnogorac‐Jurcevic, Cancer Research UK Molecular Oncology Unit, Barts and the London School of Medicine and Dentistry, London, UK
H L Yin, University of Texas Southwestern Medical Centre, Dallas, Texas, USACorrespondence to: E Costello Division of Surgery and Oncology, Royal Liverpool University Hospital, 5th Floor UCD Building, Daulby Street, University of Liverpool, Liverpool L68 3 GA, UK; ecostell@liv.ac.uk
*These authors contributed equally to this work.
PMID: 16847067 PMCID: PMC1856675DOI: 10.1136/gut.2005.083691
Problematic data figures 3A and 6A. Much more similar than expected.
LikeLike
Oncogene. 2004 Mar 11;23(10):1911-21. doi: 10.1038/sj.onc.1207318.
The ATR-p53 pathway is suppressed in noncycling normal and malignant lymphocytes
Gillian G Jones 1, Philip M Reaper, Andrew R Pettitt, Paul D Sherrington
Affiliations collapse
Affiliation
1Department of Haematology, Royal Liverpool University Hospital, Prescot Street, Liverpool L7 8XP, UK.
PMID: 14755251
DOI: 10.1038/sj.onc.1207318
Figures 1 and 2a. Much more similar than expected.
https://pubpeer.com/publications/CBC6B0C731D49A437C2270EF5BA2CE#1
https://imgur.com/3vlssfR
Figure 2c. Much more similar than expected.
https://pubpeer.com/publications/CBC6B0C731D49A437C2270EF5BA2CE#2
https://imgur.com/ajFDn8r
LikeLike
Leukemia. 2005 Apr;19(4):586-94. doi: 10.1038/sj.leu.2403653.
PI3-kinase/Akt is constitutively active in primary acute myeloid leukaemia cells and regulates survival and chemoresistance via NF-kappaB, Mapkinase and p53 pathways
V L Grandage 1, R E Gale, D C Linch, A Khwaja
Affiliation
1
Royal Free and University College London Medical School, Department of Haematology, 98 Chenies Mews, London, UK. vlg@talk21.com
PMID: 15703783 DOI: 10.1038/sj.leu.2403653
Problematic data figure 1. Much more similar than expected.
LikeLike
Oncogene. 2004 Jul 29;23(34):5864-70. doi: 10.1038/sj.onc.1207711.
Human papillomavirus type 77 E6 protein selectively inhibits p53-dependent transcription of proapoptotic genes following UV-B irradiation
Silvia Giampieri 1, Ramon García-Escudero, Judith Green, Alan Storey
Affiliation
1Cancer Research UK, Skin Tumour Laboratory, 2 Newark Street, London E1 2AT, UK.
PMID: 15077176 DOI: 10.1038/sj.onc.1207711
Highly problematic data figure 1a. Much more similar than expected.
LikeLike
Neuro Oncol. 2011 May;13(5):487-99. doi: 10.1093/neuonc/nor010. Epub 2011 Mar 1.
Rad51 inhibition is an effective means of targeting DNA repair in glioma models and CD133+ tumor-derived cells
Susan C Short 1, Silvia Giampieri, Mulugeta Worku, Marisa Alcaide-German, George Sioftanos, Sara Bourne, Ka Ian Lio, Maya Shaked-Rabi, Christine Martindale
Affiliation1UCL Cancer Institute, University College London, London, UK.
s.short@ucl.ac.uk
PMID: 21363882 PMCID: PMC3093331 DOI: 10.1093/neuonc/nor010
Problematic data figure 1A. Splicing in Rad51 panel, but not in other panels.
LikeLike
Mol Cell Biol. 2003 Apr;23(7):2530-42. doi: 10.1128/mcb.23.7.2530-2542.2003.
Dissecting the contribution of p16(INK4A) and the Rb family to the Ras transformed phenotype
Philip J Mitchell 1, Elena Perez-Nadales, Denise S Malcolm, Alison C Lloyd
Affiliation
1MRC Laboratory for Molecular Cell Biology and Department of Biochemistry, University College London, London WC1E 6BT, United Kingdom.
PMID: 12640134 PMCID: PMC150721 DOI: 10.1128/mcb.23.7.2530-2542.2003
Figures 3 and 4. Much more similar than expected.
https://pubpeer.com/publications/DA20D0100EC914D112C2688E0AD69E#3
LikeLike
Cancer Res. 2005 Apr 15;65(8):3035-9. doi: 10.1158/0008-5472.CAN-04-4194.
Spontaneous Human Adult Stem Cell Transformation
AUTHOR INFORMATION
Daniel Rubio1, Javier Garcia-Castro1,2, María C. Martín3, Ricardo de la Fuente1, Juan C. Cigudosa3, Alison C. Lloyd4, and Antonio Bernad1
1Department of Immunology and Oncology, Centro Nacional de Biotecnología/Consejo Superior de Investigaciones Cientificas, UAM Campus de Cantoblanco;
2Oncology Department, Hospital Universitario del Niño Jesús;
3Cytogenetics Unit, Centro Nacional de Investigaciones Oncológicas, Madrid, Spain; and
4Laboratory for Molecular Cell Biology, University College London, London, United Kingdom
Requests for reprints:
Javier Garcia-Castro, Department of Immunology, Centro Nacional de Biotechnologia/Consejo Superior de Investigaciones Cientificas, UAM Campus de Cantoblanco, Darwin, 3 E-28049 Madrid, Spain. Phone: 34-915854656; Fax: 34-913720493; E-mail: igarciac.hnjs@salud.madrid.org
2010 retraction.
https://cancerres.aacrjournals.org/content/70/16/6682
The authors retract the article titled “Spontaneous Human Adult Stem Cell Transformation,” which was published in the April 15, 2005, issue of Cancer Research (1). Upon review of the data published in this article, the authors have been unable to reproduce some of the reported spontaneous transformation events and suspect the phenomenon is due to a cross-contamination artifact. Five of the seven authors have agreed to the retraction of this paper.
Ricardo de la Fuente
Antonio Bernad
Department of Immunology and Oncology, Centro Nacional de Biotecnologia/Consejo Superior de Investigaciones Cientificas, UAM Campus de Cantoblanco, Madrid, Spain
Javier Garcia-Castro
Department of Immunology and Oncology, Centro Nacional de Biotecnologia/Consejo Superior de Investigaciones Cientificas, UAM Campus de Cantoblanco, Madrid, Spain
Oncology Department, Hospital Universitario del Nino Jesus, Madrid, Spain
Maria C. Martin
Juan C. Cigudosa
Cytogenetics Unit, Centro National de Investigaciones Oncological, Madrid, Spain
©2010 American Association for Cancer Research.
Reference
↵Rubio D, Garcia-Castro J, Martin MC, dela Fuente R, Cigudosa JC, Lloyd AC, Bernard A. Spontaneous human adult stem cell transformation. Cancer Res 2005;65:3035–9.
This paper was a big deal. Cited 1385 times!
Spontaneous human adult stem cell transformation
…, R de la Fuente, JC Cigudosa, AC Lloyd, A Bernad – Cancer research, 2005 – AACR
Human adult stem cells are being evaluated widely for various therapeutic approaches.
Several recent clinical trials have reported their safety, showing them to be highly resistant to
transformation. The clear similarities between stem cell and cancer stem cell genetic …
Cited by 1385 Related articles All 12 versions
Even more amazingly cited 758 times since the beginning of 2011!
https://scholar.google.co.uk/scholar?hl=en&as_sdt=2005&sciodt=0%2C5&cites=17580532675417096785&scipsc=&as_ylo=2011&as_yhi=2021
LikeLike
Data in Cell Rep. 2019 Feb 5;26(6):1458-1472.e4. doi: 10.1016/j.celrep.2018.12.081
from
PLoS One . 2017 Feb 24;12(2):e0172736. doi: 10.1371/journal.pone.0172736. eCollection 2017.
Much more similar and different than expected.
Cell Rep. 2019 Feb 5;26(6):1458-1472.e4 currently being discussed at Pubpeer:
https://pubpeer.com/publications/2BB1101F1420F1247D963A9EDF9909#null
Cell Rep. 2019 Feb 5;26(6):1458-1472.e4. doi: 10.1016/j.celrep.2018.12.081.
Macrophage-Derived Slit3 Controls Cell Migration and Axon Pathfinding in the Peripheral Nerve BridgeXin-Peng Dun 1, Lauren Carr 2, Patricia K Woodley 2, Riordan W Barry 3, Louisa K Drake 3, Thomas Mindos 2, Sheridan L Roberts 2, Alison C Lloyd 4, David B Parkinson 2Affiliations1Faculty of Medicine and Dentistry, Plymouth University, Plymouth, Devon, UK; School of Pharmacy, Hubei University of Science and Technology, Xian-Ning City, Hubei, China; The Co-innovation Center of Neuroregeneration, Nantong University, Jiangsu Province, China. Electronic address: xin-peng.dun@plymouth.ac.uk.2Faculty of Medicine and Dentistry, Plymouth University, Plymouth, Devon, UK.3University of Bath, Bath, UK.4MRC Laboratory for Molecular Cell Biology, University College London, London, UK.PMID: 30726731 PMCID: PMC6367597 DOI: 10.1016/j.celrep.2018.12.081
PLoS One2017 Feb 24;12(2):e0172736. doi: 10.1371/journal.pone.0172736. eCollection 2017.
Expression patterns of Slit and Robo family members in adult mouse spinal cord and peripheral nervous systemLauren Carr 1, David B Parkinson 1, Xin-Peng Dun 1 2Affiliations1Plymouth University Peninsula Schools of Medicine and Dentistry, Plymouth, Devon, United Kingdom.2Hubei University of Science and Technology, Xian-Ning City, Hubei, China.PMID: 28234971 PMCID: PMC5325304 DOI: 10.1371/journal.pone.0172736
LikeLike
Mol Pharmacol. 2003 Nov;64(5):1101-8. doi: 10.1124/mol.64.5.1101.
Acquired cellular resistance to flavopiridol in a human colon carcinoma cell line involves up-regulation of the telomerase catalytic subunit and telomere elongation. Sensitivity of resistant cells to combination treatment with a telomerase inhibitor
Christopher M Incles 1, Christoph M Schultes, Lloyd R Kelland, Stephen Neidle
Affiliation1The School of Pharmacy, 29-39 Brunswick Square, London WC1N 1AX, UK. stephen.neidle@ulsop.ac.ukPMID: 14573759 DOI: 10.1124/mol.64.5.1101
Problematic data figure 4. Much more similar than expected.
LikeLike
PLoS One. 2011;6(12):e28506. doi: 10.1371/journal.pone.0028506. Epub 2011 Dec 9.
Latent Epstein-Barr virus can inhibit apoptosis in B cells by blocking the induction of NOXA expression
Jade Yee 1, Robert E White, Emma Anderton, Martin J Allday
Affiliation1Section of Virology, Division of Infectious Diseases, Faculty of Medicine, Imperial College London, London, United Kingdom.PMID: 22174825 PMCID: PMC3235132 DOI: 10.1371/journal.pone.0028506
Problematic data figure 3C. Much more similar than expected.
LikeLike
Continuation Cell. 2005 Dec 16;123(6):1065-78. doi: 10.1016/j.cell.2005.09.032.
hnRNP K: an HDM2 target and transcriptional coactivator of p53 in response to DNA damage
Abdeladim Moumen 1, Philip Masterson, Mark J O’Connor, Stephen P Jackson
Figures 1E, 1F, and 3A. Much more similar than expected, although the conditions, cells are different.
LikeLike
Oncotarget. 2014 Sep 30;5(18):8270-83. doi: 10.18632/oncotarget.2013.
Oncogenic RAS-induced senescence in human primary thyrocytes: molecular effectors and inflammatory secretome involved
Maria Grazia Vizioli 1, Joana Santos 2, Silvana Pilotti 3, Mara Mazzoni 4, Maria Chiara Anania 4, Claudia Miranda 4, Sonia Pagliardini 4, Marco A Pierotti 5, Jesus Gil 6, Angela Greco 7
Affiliations
1Molecular Mechanism Unit, Department of Experimental Oncology and Molecular Medicine, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy. Cell Proliferation Group, MRC Clinical Sciences Centre, Imperial College London, Hammersmith Campus, London, UK.
2Cell Proliferation Group, MRC Clinical Sciences Centre, Imperial College London, Hammersmith Campus, London, UK.
3Laboratory of Molecular Pathology, Department of Pathology, IRCCS Foundation – Istituto Nazionale dei Tumori, Milan, Italy.
4Molecular Mechanism Unit, Department of Experimental Oncology and Molecular Medicine, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy.
5Scientific Directorate, IRCCS Foundation – Istituto Nazionale dei Tumori, Milan, Italy.
6Cell Proliferation Group, MRC Clinical Sciences Centre, Imperial College London, Hammersmith Campus, London, UK. Senior co-authors.
7Molecular Mechanism Unit, Department of Experimental Oncology and Molecular Medicine, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy. Senior co-authors.
PMID: 25268744 PMCID: PMC4226682 DOI: 10.18632/oncotarget.2013
Figures 1d and 2d. Much more similar (Yellow) and different (Red) than expected.
LikeLike
Mol Cell. 2013 Mar 7;49(5):858-71. doi: 10.1016/j.molcel.2013.01.002. Epub 2013 Jan 17.
RIF1 is essential for 53BP1-dependent nonhomologous end joining and suppression of DNA double-strand break resectionhttps://www.ncbi.nlm.nih.gov/pmc/articles/PMC3594748/
J. Ross Chapman,1 Patricia Barral,2 Jean-Baptiste Vannier,1 Valérie Borel,1 Martin Steger,3 Antonia Tomas-Loba,1 Alessandro A. Sartori,3 Ian R. Adams,4 Facundo D. Batista,2 and Simon J. Boulton1,∗
Author information
1DNA Damage Response Laboratory, London Research Institute, Cancer Research UK, Clare Hall, South Mimms, London EN6 3LD, UK
2Lymphocyte Interaction Group, London Research Institute, Cancer Research UK, 44 Lincoln’s Inn Field, London WC2A 3LY, UK
3Institute of Molecular Cancer Research, University of Zurich, Winterthurerstrasse 190, 8057 Zurich, Switzerland
4MRC Human Genetics Unit, MRC Institute of Genetics and Molecular Medicine, University of Edinburgh, Western General
Hospital, Crewe Road, Edinburgh EH4 2XU, UK
Simon J. Boulton: ku.gro.recnac@notluob.nomis
∗Corresponding author ku.gro.recnac@notluob.nomis
.
PMID: 23333305 PMCID: PMC3594748 DOI: 10.1016/j.molcel.2013.01.002
Figure 6. Much more similar (Blue) and different (Red) than expected.
https://pubpeer.com/publications/DCB611E3C696559B72A92088F15535
LikeLike
J Biol Chem. 2013 Nov 8;288(45):32357-32369. doi: 10.1074/jbc.M113.459164. Epub 2013 Aug
Tripartite Motif-containing 33 (TRIM33) protein functions in the poly(ADP-ribose) polymerase (PARP)-dependent DNA damage response through interaction with Amplified in Liver Cancer 1 (ALC1) protein
Atul Kulkarni 1, Jay Oza 1, Ming Yao 1, Honeah Sohail 1, Vasudeva Ginjala 1, Antonia Tomas-Loba 2, Zuzana Horejsi 2, Antoinette R Tan 1, Simon J Boulton 2, Shridar Ganesan 3
Affiliations
1From the Department of Medicine, Rutgers Cancer Institute of New Jersey, Rutgers University, New Brunswick, New Jersey 08903.
2the DNA Damage Response Laboratory, London Research Institute, Clare Hall, South Mimms, EN6 3LD Herts, United Kingdom.
3From the Department of Medicine, Rutgers Cancer Institute of New Jersey, Rutgers University, New Brunswick, New Jersey 08903. Electronic address: ganesash@umdnj.edu.
PMID: 23926104 PMCID: PMC3820871 DOI: 10.1074/jbc.M113.459164
Problematic data figure 1B. Much more similar than expected.
LikeLike
Pflugers Arch. 2010; 459(3): 451–463.
Published online 2009 Oct 13. doi: 10.1007/s00424-009-0717-4
PMCID: PMC2810363
PMID: 19823867
Lipopolysaccharide modifies amiloride-sensitive Na+ transport processes across human airway cells: role of mitogen-activated protein kinases ERK 1/2 and 5
D. L. Baines,corresponding author1 A. P. Albert,1 M. J. Hazell,2 L. Gambling,3 A. M. Woollhead,1 and M. E. C. Dockrell2
Author information
1St. George’s, University of London, Tooting, London SW17 0RE UK
2S.W. Thames Institute of Renal Research, St. Helier Hospital, Carshalton, Surrey SM5 1AA UK
3The Rowett Institute of Nutrition and Health, Bucksburn, Aberdeen AB21 9SB UK
D. L. Baines, Phone: +44-208-7250916, Fax: +44-208-7252993, Email: .d.baines@sghms.ac.uk
corresponding author.
Problematic data figures 6a and 7a.
Much more similar and different than expected.
LikeLike
J Biol Chem. 2001 Jun 29;276(26):23922-8. doi: 10.1074/jbc.M100384200. Epub 2001 Mar 23.
Disruption of the interaction of mammalian protein synthesis eukaryotic initiation factor 4B with the poly(A)-binding protein by caspase- and viral protease-mediated cleavages
M Bushell 1, W Wood, G Carpenter, V M Pain, S J Morley, M J Clemens
Affiliation
1Department of Biochemistry and Immunology, Cellular and Molecular Sciences Group, St. George’s Hospital Medical School, Cranmer Terrace, London SW17 0RE, United Kingdom.
PMID: 11274152
DOI: 10.1074/jbc.M100384200
https://pubpeer.com/publications/7FB269E1C7F53ED9093676859657A0#
Figure 2C. Much more similar than expected.
LikeLike
FEBS Lett. 2000 Jul 21;477(3):229-36. doi: 10.1016/s0014-5793(00)01805-6.
Differential requirements for caspase-8 activity in the mechanism of phosphorylation of eIF2alpha, cleavage of eIF4GI and signaling events associated with the inhibition of protein synthesis in apoptotic Jurkat T cells
S J Morley 1, I Jeffrey, M Bushell, V M Pain, M J Clemens
Affiliation
1Biochemistry Laboratory, School of Biological Sciences, University of Sussex, Falmer, Brighton BN1 9QG, UK. s.j.morely@sussex.ac.uk
PMID: 10908726
DOI: 10.1016/s0014-5793(00)01805-6
Figure 4A. Much more similar after horizontal flip than expected.
https://pubpeer.com/publications/41E163998740C2E66E3B0D40D5B3A5
LikeLike
Hum Gene Ther. 2013 Jul;24(7):692-701. doi: 10.1089/hum.2013.081.
Gene correction of a duchenne muscular dystrophy mutation by meganuclease-enhanced exon knock-in
Linda Popplewell 1, Taeyoung Koo, Xavier Leclerc, Aymeric Duclert, Kamel Mamchaoui, Agnés Gouble, Vincent Mouly, Thomas Voit, Frédéric Pâques, Frédéric Cédrone, Olga Isman, Rafael J Yáñez-Muñoz, George Dickson
Affiliation
1School of Biological Sciences, Royal Holloway University of London, Egham, Surrey TW20 0EX, United Kingdom.
PMID: 23790397 DOI: 10.1089/hum.2013.081
Figure 2a.
2 days and 5 days post-infection blots much more similar than expected.
LikeLike