The Danish cancer researcher and entrepreneur Janine Erler retracted her 14 year old Nature paper. It was the product of her postdoctoral studies at Stanford University, USA, which catapulted her stellar career in academia and business. I presented Erler’s story in my previous article in 2018, together with the evidence of excessive data manipulation in the 2006 Nature publication.
The retraction of Erler et al Nature 2006 on 18 March 2020 was a major surprise. Stanford namely officially closed the case and told everyone who kept complaining to get lost. Maybe Nature really began a new research integrity policy? In this particular case, the claims of a lysil oxydase (LOX) inhibitors to being a cure for cancer never went anywhere in clinical trials. Labs whose LOX research allegedly supports Erler’s results, like labs of Richard Marais and Caroline Springer, stand accused of bad scientific practices themselves. In short, it very may be that the entire billion-dollar-heavy LOX field was a cancerous hoax.

To put things into the correct perspective, the ultimate US authority on research integrity, Retraction Watch, now brought an article about Erler’s Nature retraction. Stanford is mentioned only once in passing and, most notably: Erler’s former mentor and the paper’s last author Amato Giaccia is not mentioned even once. As if he has absolutely nothing to do with any of that unfortunate cock-up.
That is a pity, because Giaccia’s lab published a number of problematic papers without Erler’s contribution. Clare Francis, who posted some evidence years ago on PubPeer, kindly agreed to have yet another look. It seems, Giaccia is simultaneously very famous and revered cancer researcher, but also a somewhat bad principal investigator, of the kind who lets his lab members publish fake data and does nothing to fix the literature. The main conclusions, that everyone made a nice academic career and earned a lot of money (especially Giaccia himself), do remain unaffected indeed, so there. Only right that the Watchdogs of Retraction Watch decided not to burden their readers’ gentle brains with any mention of this Italian cancer researcher.
Giaccia has since left Stanford and joined another highly prestigious institution, the University of Oxford in UK, where he now heads the MRC Oxford Institute for Radiation Oncology. Which is the absolutely right place to seek shelter with when you are caught on rigged science. At Oxford, data manipulation is never investigated if the paper is older than 3 years and/or if the author is very important (see Oxford’s statement on the case of Dame Kay Davies).
The MRC issued in January 2019 a press release celebrating Giaccia’s appointment:
“Amato is a fantastic catch for the Oxford oncology community and we are delighted he has chosen to join us from Stanford. He has already made his presence felt having hit the ground running and we look forward to him taking the Institute to the next level.”
It is a challenge to take Oxford’s Photoshop output to the next level, but Giaccia is definitely the right man for that. Let’s have a look what his Stanford lab delivered under his guidance. As starter, a collaborative paper with the Oxford MRC Institute for Radiation Oncology, published 10 years before Giaccia become its director:

Z Bencokova , MR Kaufmann , IM Pires , PS Lecane , AJ Giaccia , EM Hammond ATM activation and signaling under hypoxic conditions Molecular and Cellular Biology (2009) doi: 10.1128/mcb.01301-08
Incidentally, the last author and Oxford professor Esther Hammond is a former Giaccia mentee, she did postdoc in his Stanford lab. The PubPeer evidence is festering for already 3 years. Yet these two Oxford professors have better things to do, like getting hold of more of your tax and charity money for quality cancer research.
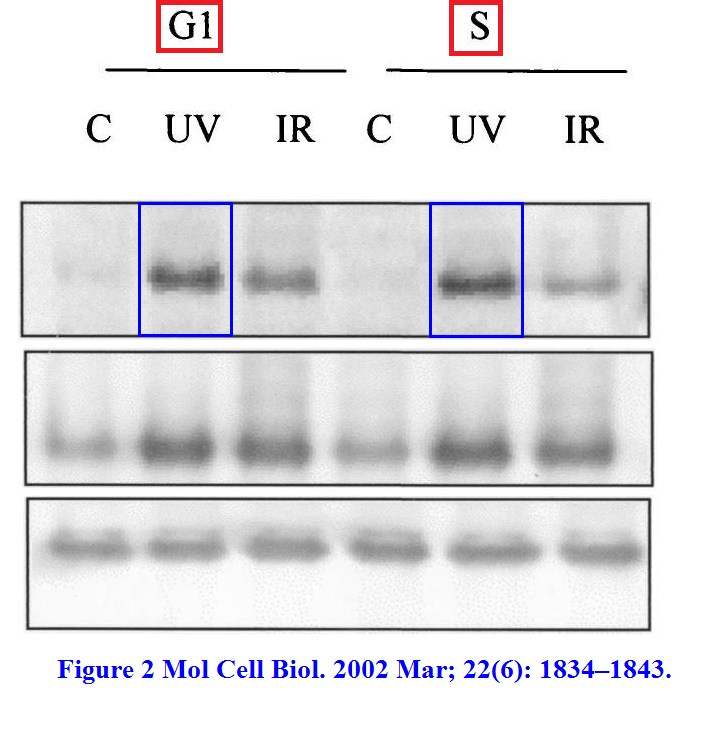
You probably started to pity poor Dr Hammond for becoming victim of someone else’s naughtiness? That is from her own first author paper as Giaccia’s postdoc:
EM. Hammond, NC. Denko, MJ Dorie, RT. Abraham, AJ. Giaccia Hypoxia Links ATR and p53 through Replication Arrest Molecular and Cellular Biology (2002) doi: 10.1128/MCB.22.6.1834-1843.2002
Another Giaccia classic, 20 years old, Clare Francis even spotted a dog’s face there, twice:
H L Maecker, C Koumenis, A J Giaccia p53 promotes selection for Fas-mediated apoptotic resistance Cancer Research (2000) Aug 15;60(16):4638-44.


Cloned gel lanes? Woof woof to that. Here is a vintage one, by now 22 years old and same age as many of my readers. A cloned and mirrored gel band:
Zundel W, Giaccia A. Inhibition of the anti-apoptotic PI(3)K/Akt/Bad pathway by stress. Genes & Development (1998) DOI: 10.1101/gad.12.13.1941

If you liked that, here is another original Zundel for you:
Zundel W, Swiersz LM, Giaccia A. Caveolin 1-mediated regulation of receptor tyrosine kinase-associated phosphatidylinositol 3-kinase activity by ceramide.Molecular and Cellular Biology (2000) DOI: 10.1128/mcb.20.5.1507-1514.2000
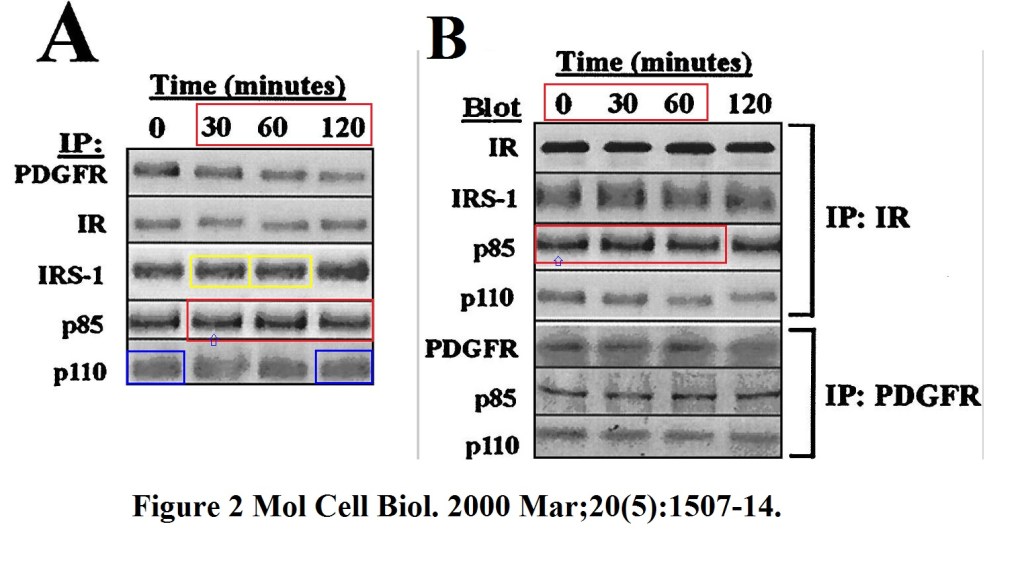
Pretty, isn’t it? That Wayne Zundel apparently was really determined to make a career in academia. He had several stints as department head at University of Louissville and Oregon Health & Sciences University, until he opened his own consultancy business in 2011. Is this the kind of scientific method Zundel now consults about? Will Zundel be invited to Oxford Radiation Oncology to show the tricks to young aspiring graduate students and postdocs there? One more:
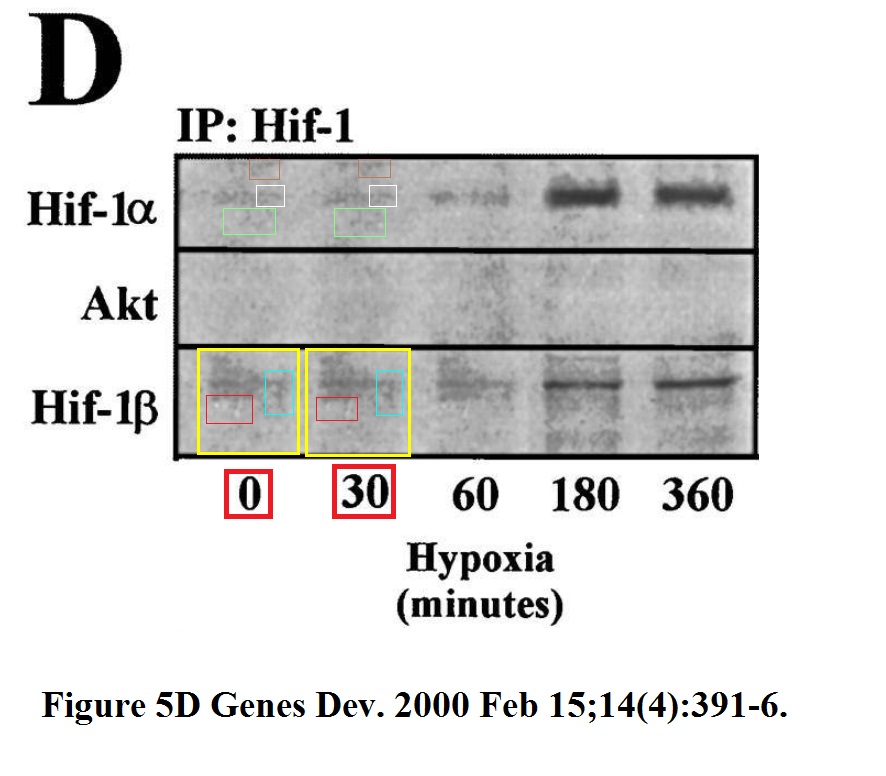
W Zundel , C Schindler , D Haas-Kogan , A Koong , F Kaper , E Chen , A R Gottschalk , H E Ryan , R S Johnson , A B Jefferson , D Stokoe , AJ Giaccia Loss of PTEN facilitates HIF-1-mediated gene expression Genes & Development (2000) Feb 15;14(4):391-6.
Here a collaborative study featuring Giaccia and other Stanford colleagues. The first author Barbara Bedogni now runs a lab at the University of Miami:
Bedogni B, Warneke JA, Nickoloff BJ, Giaccia AJ, Powell MB. Notch1 is an effector of Akt and hypoxia in melanoma development. J Clin Invest (2008) DOI: 10.1172/JCI36157
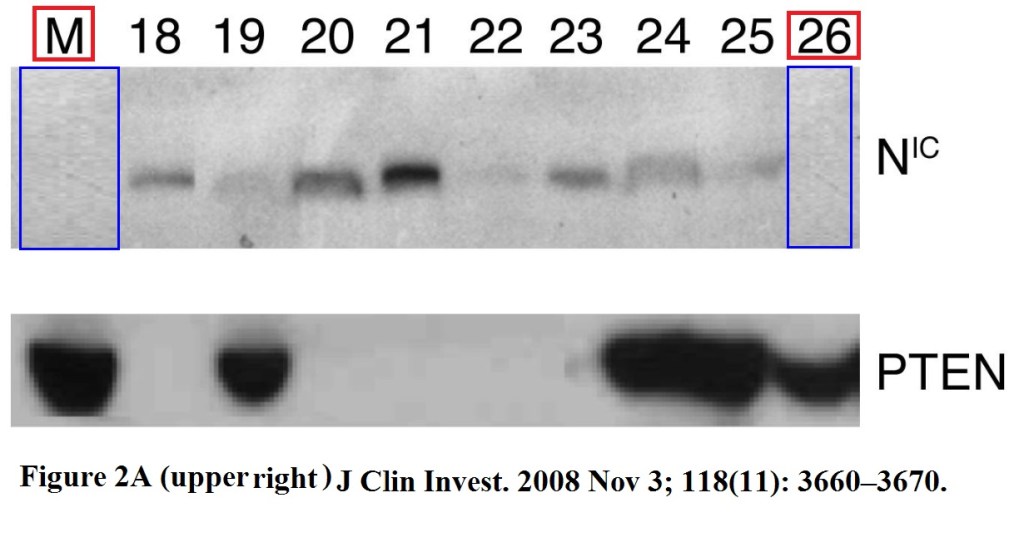

As you see, it is not always just duplications, but also many spliced gels. A legend goes around in biomedicine that it was once “legal” until 2008 to stealthily splice lanes, in order to bullshit readers into believing they were looking at an intact gel, truthfully probed for equal loading. Luckily, Giaccia published that spliced paper just in 2008, and then another one in Cancer Cell, which is nice because at Cell Press nobody cares about data manipulation anyway.
S Turcotte , DA. Chan , PD. Sutphin , MP. Hay , WA. Denny , AJ. Giaccia A molecule targeting VHL-deficient renal cell carcinoma that induces autophagy Cancer Cell (2008) doi: 10.1016/j.ccr.2008.06.004
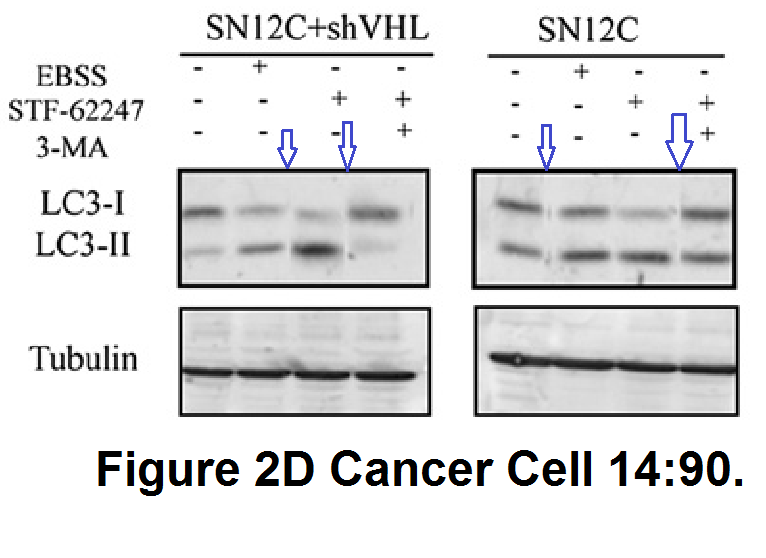
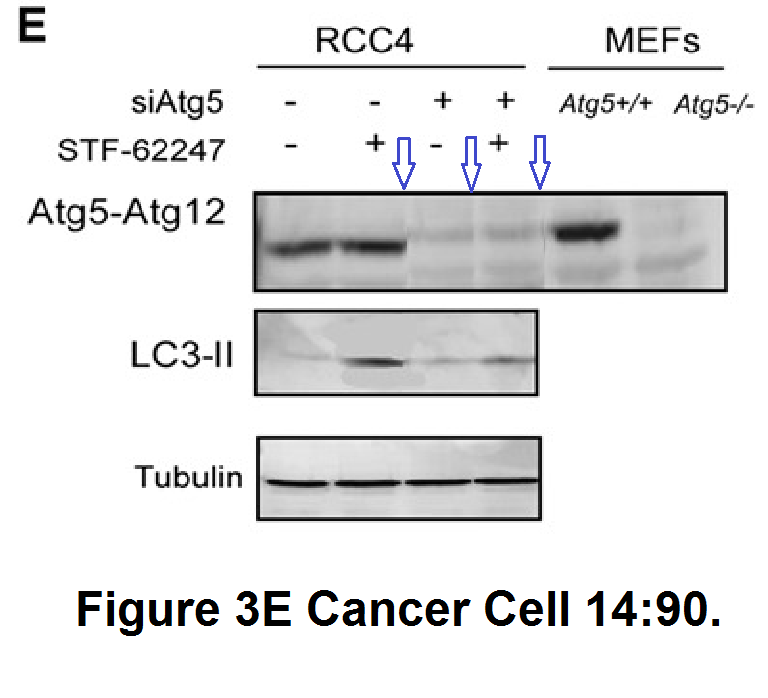
What exactly is the scientific message of this collage? Not much remains standing, except to wonder why authors resorted to such tricks. But it helped Sandra Turcotte become professor at the Atlantic Cancer Research Institute in Canada. Sure, 2008, the mythical deadline, state of limitations etc. But this Giaccia paper is from 2014:
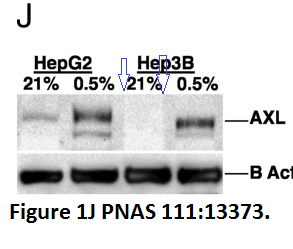
EB. Rankin, KC. Fuh, L Castellini, K Viswanathan, EC. Finger, AN. Diep, EL. LaGory, MS. Kariolis, A Chan, D Lindgren, H Axelson, YR. Miao, AJ. Krieg, AJ. Giaccia Direct regulation of GAS6/AXL signaling by HIF promotes renal metastasis through SRC and MET Proceedings of the National Academy of Sciences (2014) doi: 10.1073/pnas.1404848111
Now, it again may look like nothing, but two things are clear: The 3rd lane of the AXL western blot was digitally spliced in, and the entire analysis lacks a proper loading control. The reason why PNAS has done absolutely nothing about that so far is the fact that Giaccia is such a bigwig that you simply must trust him on his word. The paper was incidentally editorially handled by Tony Hunter, star of the Salk Institute who is also too big to bother about what he actually published.
Now I would like to introduce to you another Giaccia alumnus: Cullen Taniguchi, now head of Radiation oncology at MD Anderson. In the world of cancer research, where data manipulation is boringly normal, MD Anderson stands apart because of their apparent institutional policy to actively recruit dishonest scientists. The only people MD Anderson does crack down upon in research fraud cases, are the whistleblowers. Here is what Taniguchi published with Giaccia:
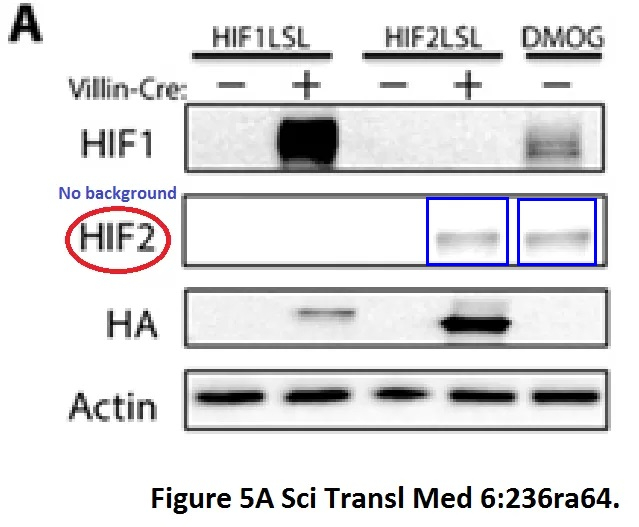
CM Taniguchi, YR Miao, AN Diep, C Wu, EB Rankin, TF Atwood, L Xing, AJ Giaccia PHD inhibition mitigates and protects against radiation-induced gastrointestinal toxicity via HIF2 Science Translational Medicine (2014) doi: 10.1126/scitranslmed.3008523
Not convinced about these two bands? Have a look at another paper by Taniguchi which qualified him for the MD Anderson faculty job:
CM Taniguchi , EC Finger , AJ Krieg , C Wu , AN Diep , EL LaGory , K Wei , LM McGinnis , J Yuan , CJ Kuo , AJ Giaccia Cross-talk between hypoxia and insulin signaling through Phd3 regulates hepatic glucose and lipid metabolism and ameliorates diabetes Nature Medicine (2013) doi: 10.1038/nm.3294
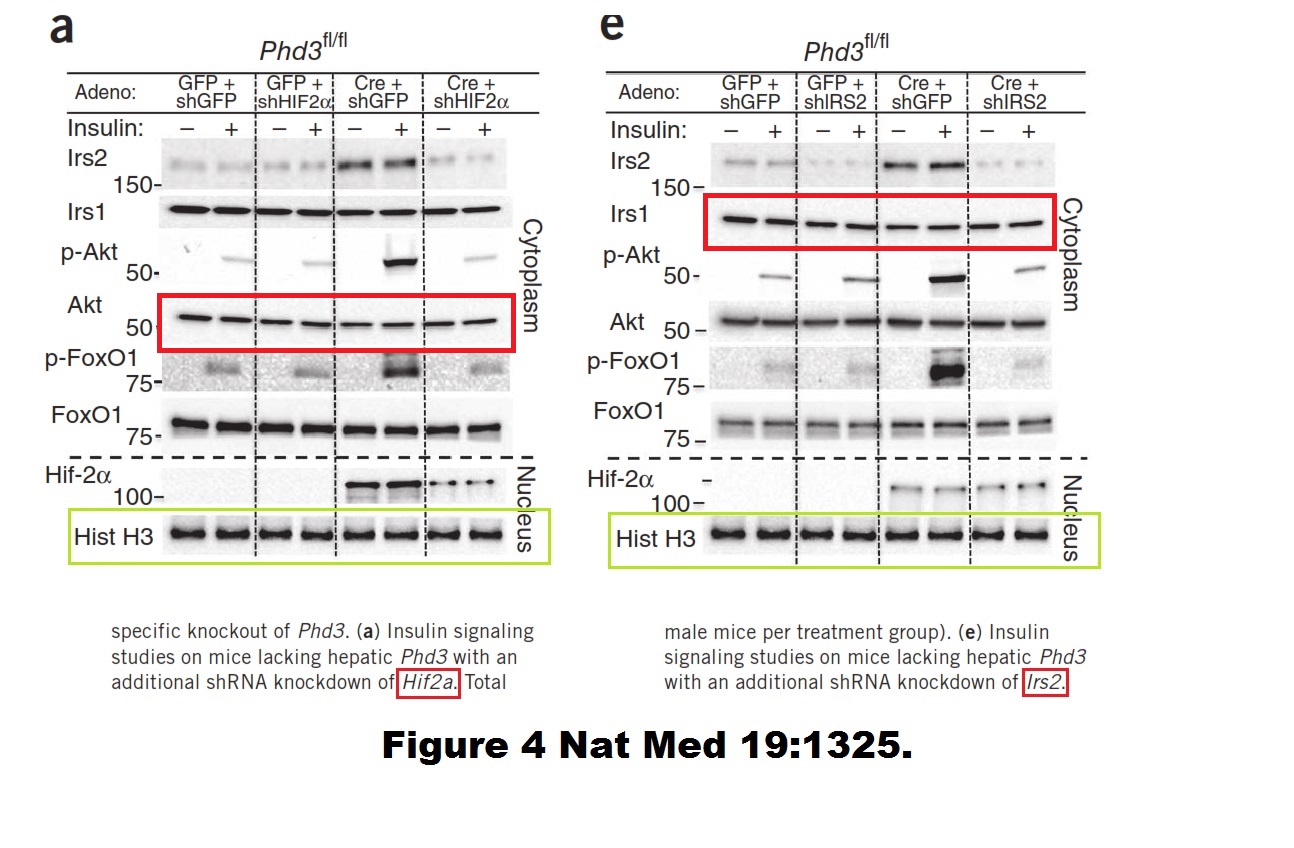
Still thinking about innocent oversight as possible explanation? Taniguchi is the middle one on this 3-author paper, the last one being the Harvard star scientist C Ronald Kahn, another American giant who can’t be bothered to apply a minimum of research integrity to his groundbreaking biomedical research. The paper Entingh et al 2003 has been retracted by the no-nonsense Journal of Biological Chemistry:
For Retraction Watch, Kahn explained that the results of the retracted study were perfectly reproducible, while Taniguchi surprised everyone that he actually contributed no research data whatsoever to that paper. Probably like with the other 6 papers with Kahn, flagged on Pubpeer for data manipulation. Which might be even true, Taniguchi likely only contributed some artwork instead. Like this Taniguchi et al Mol Cell Biol 2007:

Scientist titans like Kahn and Giaccia are the reason why USA is the scientific world leader, followed by United Kingdom. But China is catching up.
Update 20.04.2020
Oxford University Registrar Gillian Aitken predictably refused outright a research misconduct investigation, just as she did in a different case (that of Kay Davies). I received this reply:
“I have considered whether the matter should be reviewed under the University’s Code of practice and procedure on academic integrity in research, and have concluded that as the papers were published before Professor Giaccia was employed by the University, and more than three years ago, the University’s Code is not engaged.
I have therefore decided that no further investigation is required by the University of Oxford, and I consider this matter closed.“

Donate!
If you are interested to support my work, you can leave here a small tip of $5. Or several of small tips, just increase the amount as you like (2x=€10; 5x=€25). Your generous patronage of my journalism will be most appreciated!
€5.00



“Now I would like to introduce to you another Giaccia alumnus: Cullen Taniguchi, now head of Radiation oncology at MD Anderson.”
I might be missing something.
J Clin Invest. 2005 Mar;115(3):718-27.
Complementary roles of IRS-1 and IRS-2 in the hepatic regulation of metabolism.
Taniguchi CM1, Ueki K, Kahn R.
Author information
1
Research Division, Joslin Diabetes Center, Harvard Medical School, Boston, Massachusetts, USA.
1st November 2016 retraction notice.
https://www.jci.org/articles/view/90689
“At the request of the corresponding author, the JCI is retracting this article. The authors were recently made aware of duplicated bands in Figures 1B, 3C, and 4C. After an extensive internal review, it was discovered that these duplications were introduced during figure assembly. The authors have stated that experimental data generated in the lab from the same time period support the original conclusions of the study and that other studies have subsequently confirmed and extended the primary conclusions of the manuscript. However, in the interest of maintaining accuracy in the published scientific literature and because the initial figures were not up to the standards of the JCI, the authors wish to retract this article. The authors apologize for these errors.”
LikeLike
https://mrc.ukri.org/news/browse/new-director-of-mrc-oxford-institute-for-radiation-oncology/
Dr Mariana Delfino-Machin, MRC Programme Manager for Cancer, said: “OIRO is the MRC’s major strategic investment in radiation oncology research and the appointment of Professor Giaccia provides a platform for OIRO to build upon its significant discoveries in the field. Professor Giaccia is an excellent addition to the UK’s radiation oncology community.”
How can Dr Mariana Delfino-Machin tell if “Professor Giaccia is an excellent addition to the UK’s radiation oncology community”? What is her evidence, or experience? Who told her to say those things (another manager)? Is it wishful thinking, or simply window-dressing?
https://www.ncbi.nlm.nih.gov/pubmed/?term=delfino-machin
According to Pubmed Dr Mariana Delfino-Machin has 6 publications about development, and 1 for counting creatures living in estuaries.
https://www.ncbi.nlm.nih.gov/pubmed/?term=delfino-machin
Why be a scientist if you can be a manager of scientists, or is that mean to write?
LikeLike
Delfino-Machin may have been a post-doc that was promoted to this position by someone that she worked for. I see this all of the time in the institution I work. Here, for this kind of internal position, what gets you the job is who you know.
Having Snodfart, oops, I mean Stanford on your resume is like having Harvard: even if you didn’t publish much, that work will make doors fly open for you. “oooooohhh….this person must be smart!” That kind of thing. Heck, whats even more impressive is if you are an undergraduate who drops out of Stanford, like Elizabeth Holmes. “oooohhh….she’s the next Steve Jobs!!!” sigh
LikeLike
“Elizabeth Holmes. “oooohhh….she’s the next Steve Jobs!!!” sigh”
Still not gone to prison even though her second baby was born months ago
Must be a rich, blonde, white woman thing.
LikeLike
“W Zundel , C Schindler , D Haas-Kogan , A Koong , F Kaper , E Chen , A R Gottschalk , H E Ryan , R S Johnson , A B Jefferson , D Stokoe , AJ Giaccia Loss of PTEN facilitates HIF-1-mediated gene expression Genes & Development (2000) Feb 15;14(4):391-6.”
Fresh air destroying Roman frescoes. Don’t let the oxygen in!
Fresh air destroying figure 5D. Don’t let the oxygen in!
LikeLike
Oxford blindspot. None of the authors noticed anything.
Cancer Cell. 2013 May 13;23(5):618-33. doi: 10.1016/j.ccr.2013.03.013. Epub 2013 Apr 25.
Restoring p53 function in human melanoma cells by inhibiting MDM2 and cyclin B1/CDK1-phosphorylated nuclear iASPP.
Lu M1, Breyssens H, Salter V, Zhong S, Hu Y, Baer C, Ratnayaka I, Sullivan A, Brown NR, Endicott J, Knapp S, Kessler BM, Middleton MR, Siebold C, Jones EY, Sviderskaya EV, Cebon J, John T, Caballero OL, Goding CR, Lu X.
Author information
1
Ludwig Institute for Cancer Research, University of Oxford, Oxford OX3 7DQ, UK.
Figure 4G.
https://pubpeer.com/publications/7405B9B25BA9D4C140E294D40564CB#2
LikeLike
Oncogene. 2005 May 26;24(23):3836-41.
Mdm2 and mdmX prevent ASPP1 and ASPP2 from stimulating p53 without targeting p53 for degradation.
Bergamaschi D1, Samuels Y, Zhong S, Lu X.
Author information
1
Ludwig Institute for Cancer Research, University College London, 91 Riding House Street, London W1W 7BS, UK.
Figure 3a.
Figure 4c.
LikeLike
Cancer Res. 2004 Jul 15;64(14):4749-54.
Ser392 phosphorylation regulates the oncogenic function of mutant p53.
Yap DB1, Hsieh JK, Zhong S, Heath V, Gusterson B, Crook T, Lu X.
Author information
1
Ludwig Institute for Cancer Research, St. Mary’s Branch, Norfolk Place, London W2 1PG, United Kingdom.
https://pubpeer.com/publications/60A9BF6FC939AC0153A1F423133658
Figures 4D and 5A.
LikeLike
https://www.ndm.ox.ac.uk/principal-investigators/researcher/xin-lu
https://www.ludwig.ox.ac.uk/research/xin-lu-group-page
Nat Genet. 2006 Oct;38(10):1133-41. Epub 2006 Sep 10.
iASPP preferentially binds p53 proline-rich region and modulates apoptotic function of codon 72-polymorphic p53.
Bergamaschi D1, Samuels Y, Sullivan A, Zvelebil M, Breyssens H, Bisso A, Del Sal G, Syed N, Smith P, Gasco M, Crook T, Lu X.
Author information
1
Ludwig Institute for Cancer Research, University College London, 91 Riding House Street, London W1W 7BS, UK.
https://pubpeer.com/publications/858FBC108F303612266C62378E71C5
Figure 3b.
Figure 6b.
Figure 6c.
LikeLike
Mol Cell Biol. 2004 Feb;24(3):1341-50.
ASPP1 and ASPP2: common activators of p53 family members.
Bergamaschi D1, Samuels Y, Jin B, Duraisingham S, Crook T, Lu X.
Author information
1
Ludwig Institute for Cancer Research, Imperial College School of Medicine, St. Mary’s Campus, London W2 1PG, United Kingdom.
https://pubpeer.com/publications/46CD919799986C18D15745C778C13C
Figure 5B.
LikeLike
Continuation Mol Cell Biol. 2004 Feb;24(3):1341-50.
Figure 4B.
LikeLike
Proc Natl Acad Sci U S A. 2013 Apr 30;110(18):7300-5. doi: 10.1073/pnas.1214498110. Epub 2013 Apr 15.
Role of p63 and the Notch pathway in cochlea development and sensorineural deafness.
Terrinoni A1, Serra V, Bruno E, Strasser A, Valente E, Flores ER, van Bokhoven H, Lu X, Knight RA, Melino G.
A 2014 correction has been published for this paper.
http://www.pnas.org/content/111/7/2854.full
The authors note that “Thanks to an alert reader, we noticed that in Fig. 3D, the control ChIP for MDM2 erroneously duplicated a p53-RE-III panel from an earlier paper (1). We thank the reader for bringing this issue to our attention, and we deeply apologize to the scientific community for the error.”
Edited by Michael Karin, University of California, San Diego School of Medicine, La Jolla, CA, and approved March 20, 2013 (received for review August 22, 2012)
Small selection for Michael Karin:-
https://pubpeer.com/publications/549ECA180E3177C27CEF1A5B29186B
https://pubpeer.com/publications/4966AA09CF15E616FF386E8643BE34
https://pubpeer.com/publications/70B63BF42E7304DA3CF2B81A9ACBA0
https://pubpeer.com/publications/26BF1A9A41412947E05D956E91F161
https://www.pnas.org/content/110/18/7300/tab-article-info
Author Information
Alessandro Terrinonia,1,2, Valeria Serraa,1, Ernesto Brunob, Andreas Strasserc,d, Elizabeth Valentec,d, Elsa R. Florese, Hans van Bokhovenf, Xin Lug, Richard A. Knighth, and Gerry Melinoa,h,2
aBiochemistry Laboratory Istituto Dermopatico Dell’Immacolata, c/o Department of Experimental Medicine and Surgery, University of Rome “Tor Vergata,” 00133 Rome, Italy;
bDepartment of Clinical Sciences and Translational Medicine, University of Rome “Tor Vergata,” 00133 Rome, Italy;
cThe Walter and Eliza Hall Institute of Medical Research, Parkville, VIC 3052, Australia;
dDepartment of Medical Biology, Melbourne University, Parkville, VIC 3052, Australia;
eDivision of Basic Science Research, Department of Biochemistry and Molecular Biology, University of Texas M. D. Anderson Cancer Center, Houston, TX 77030;
fDepartment of Human Genetics, Nijmegen Centre for Molecular Life Sciences, Radboud University Nijmegen Medical Centre, 6500 HB, Nijmegen, The Netherlands;
gNuffield Department of Clinical Medicine, Ludwig Institute for Cancer Research, University of Oxford, Oxford OX3 7DQ, United Kingdom; and
hToxicology Unit, Medical Research Council, Leicester University, Leicester LE1 9HN, United Kingdom
LikeLike
oncology reports 25: 1713-1719, 2011
Resveratrol induces apoptosis in breast cancer cells
by E2F1-mediated up-regulation of ASPP1
Yujie Shi1,2, Shihe Yang2 , Sandi Troup2 , Xin Lu3 , Steve Callaghan4 , David S. Park4 , Ying Xing1
and Xiaohe Yang2
1 Department of Physiology, College of Basic Medical Science, Zhengzhou University, Zhengzhou
450001, Henan, P.R. China;
2
Department of Pathology, University of Oklahoma Health Sciences Center,
Oklahoma City, OK 73104, USA;
3
Nuffield Department of Clinical Medicine, Ludwig Institute for Cancer
Research, University of Oxford, Oxford OX3 7DQ, UK;
4
Cellular Molecular Medicine, Ottawa Hospital
Research Institute, University of Ottawa, 451 Smyth Road, Ottawa, Ontario K1H 8M5, Canada
Received December 8, 2010; Accepted February 1, 2011
DOI: 10.3892/or.2011.1248
https://pubpeer.com/publications/21479363
Figure 3C.
LikeLike
Hepatology. 2010 Jan;51(1):142-53. doi: 10.1002/hep.23247.
Epigenetic silence of ankyrin-repeat-containing, SH3-domain-containing, and proline-rich-region- containing protein 1 (ASPP1) and ASPP2 genes promotes tumor growth in hepatitis B virus-positive hepatocellular carcinoma.
https://aasldpubs.onlinelibrary.wiley.com/doi/pdf/10.1002/hep.23247
Jian Zhao,1,2* Guobin Wu,1,3* Fangfang Bu,1 * Bin Lu,1 Anmin Liang,3 Lei Cao,1 Xin Tong,1 Xin Lu,4 Mengchao Wu,1 and Yajun Guo1,2
From the 1International Joint Cancer Institute & Eastern Hospital of Hepatobiliary Surgery, Second Military Medical University, Shanghai, China;
2School of
Pharmacy and Antibody Engineering Center of Ministry of Education, Shanghai Jiao Tong University and Shanghai Key Laboratory for Cell Engineering and Antibody,
Shanghai, China;
3Guangxi Cancer Hospital, Guangxi Medical University, Guangxi, China;
4Ludwig Institute for Cancer Research, University of Oxford, Nuffield Department of Clinical Medicine, Oxford, UK.
https://pubpeer.com/publications/20034025
Figure 2B.
LikeLike
PLoS Pathog. 2007 Feb; 3(2): e17.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1803007/
A New Inhibitor of Apoptosis from Vaccinia Virus and Eukaryotes
Caroline Gubser,1,* Daniele Bergamaschi,2,¤ Michael Hollinshead,1 Xin Lu,2 Frank J. M van Kuppeveld,3 and Geoffrey L Smith1,*
1 Department of Virology, Faculty of Medicine, Imperial College London, London, United Kingdom
2 Ludwig Institute for Cancer Research, Faculty of Medicine, Imperial College London, London, United Kingdom
3 Department of Medical Microbiology, Nijmegen Centre for Molecular Life Sciences, Radboud University Nijmegen Medical Centre, Nijmegen, The Netherlands
University of Texas Southwestern Medical Center, United States of America.
https://pubpeer.com/publications/08D0ABC9985315B36CF7AE06A920A1
Figure 5A.
LikeLike
Nat Genet. 2003 Feb;33(2):162-7. Epub 2003 Jan 13.
iASPP oncoprotein is a key inhibitor of p53 conserved from worm to human.
Bergamaschi D1, Samuels Y, O’Neil NJ, Trigiante G, Crook T, Hsieh JK, O’Connor DJ, Zhong S, Campargue I, Tomlinson ML, Kuwabara PE, Lu X.
Author information
1
Ludwig Institute for Cancer Research, Imperial College School of Medicine, St. Mary’s Campus, Norfolk Place, London, W2 1PG, UK.
https://pubpeer.com/publications/06080F7D25D1A1B598F4DE8DEF5238
Figure 1c.
LikeLike
From the Xin Lu stable.
J Exp Med. 2013 Mar 11;210(3):581-603. doi: 10.1084/jem.20121439. Epub 2013 Feb 18.
p63 is an alternative p53 repressor in melanoma that confers chemoresistance and a poor prognosis.
Matin RN1, Chikh A, Chong SL, Mesher D, Graf M, Sanza’ P, Senatore V, Scatolini M, Moretti F, Leigh IM, Proby CM, Costanzo A, Chiorino G, Cerio R, Harwood CA, Bergamaschi D.
Author information
1
Centre for Cutaneous Research, Blizard Institute, Barts and The London School of Medicine and Dentistry, Queen Mary University of London, London E1 2AT, England, UK.
Figure 4K.
Figure 4G.
LikeLike
From the Xin Lu stable.
J Cell Sci. 2014 Jul 15;127(Pt 14):3079-93. doi: 10.1242/jcs.144816. Epub 2014 Apr 28.
iASPP is a novel autophagy inhibitor in keratinocytes.
Chikh A1, Sanzà P1, Raimondi C2, Akinduro O1, Warnes G3, Chiorino G4, Byrne C1, Harwood CA1, Bergamaschi D5.
Author information
1
Centre for Cutaneous Research, Blizard Institute, Barts and The London School of Medicine and Dentistry, Queen Mary University of London, London E1 2AT, UK.
2
Centre for Diabetes, Blizard Institute, Barts and The London School of Medicine and Dentistry, Queen Mary University of London, London E1 2AT, UK.
3
Flow Cytometry Core Facility, Blizard Institute, Barts and The London School of Medicine and Dentistry, Queen Mary University of London, London E1 2AT, UK.
4
Cancer Genomic Laboratory, Edo ed Elvo Tempia Foundation, 13900 Biella, Italy.
5
Centre for Cutaneous Research, Blizard Institute, Barts and The London School of Medicine and Dentistry, Queen Mary University of London, London E1 2AT, UK.
https://pubpeer.com/publications/43FB16ED19FAEB3AEB42F460FFCC66
Much more similar than you would expect.
Figure 1E.
Figure 1D.
LikeLike
Pingback: Science misconduct – For Better Science
Pingback: Münster charges Jens Schwamborn’s mentor Andreas Püschel with research misconduct, again – For Better Science
Pingback: Antonio Giordano and the Sbarro Pizza Temple – For Better Science
Pingback: mTOR: conclusions not affected? – For Better Science
Pingback: The English science supremacy – For Better Science