The European Commission now took 40 days to deny my second Freedom of Information (FOI) Inquiry about the TETRA phase 2 clinical trial with cadaveric trachea transplant they are currently financing with €7 Million. This is EU’s second attempt to become world-leading manufacturer of industrial trachea transplants, after the €5mn Biotrachea led by the scandal surgeon Paolo Macchiarini was terminated mid-term. No, not because EU had any concerns for the patients, quite the opposite: he was given a clean ethics vote to go ahead. Biotrachea was terminated by the EU because the plastic tracheas Macchiarini wanted to use lacked novelty, as the documents I obtained revealed.
The TETRA trial, led by Macchiarini’s past collaborator, the UCL laryngologist Martin Birchall, was already on the brink of being terminated in the wake of the Macchiarini scandal, as EU previously indicated to me. Now, exactly the opposite happens. The trial is being prepared at full speed despite the fact that its predecessor phase 1 trial INSPIRE was suspended (because of my reporting), never recruited any of its four patients and it most likely never will. That trial is also led by Birchall (details here), it is likely that its Innovate UK funding has ran out meanwhile. EU however seems to signal that they will go ahead with phase 2 trial even if phase 1 never happens. After all, there are those 10 patients who received a cadaveric trachea transplant (here and below) and were operated under hospital exemptions between 2008 and 2012 by Macchiarini and Birchall. At least half of these 10 are dead, the lucky survivors either had their graft removed or live with permanently installed stents to prevent their rotting airways from collapsing (INSPIRE’s and TETRA’s clinical promise is actually that the patients will never need a stent). But this disaster seems exactly the reason for EU to try it again, and on a much, much bigger scale: 48 patients are scheduled to receive cadaveric tracheas. Probably because it will create employment.
The EU spokesperson ceased long ago answering my emails, after I declined to be instructed over the phone (strictly off-the-record) why EU’s approach to trachea transplanting is right; this is why I had to resort to FOI. The official time limit to answer my FOI inquiry from July 1st 2017 was 15 days, but the EU first pretended not to have received my postal address, then said they need more time, then said they need extra time to assemble the documents for me, and finally, the Director-General of the European Commission, Robert-Jan Smits, wrote to me on September 11th. He basically told me again to get lost and that he will never release any information (read here his past rejection of my FOI inquiry). His reasons, as before: the trachea transplant trial is a business enterprise and revealing any of its progress might endanger the financial interests of its stakeholders, and then there are privacy concerns. Exactly, Smits decided that the public must under no circumstances find out whom exactly the EU is giving this public’s money for research on humans. I am not making it up, read Smits’ letter yourself here.

Here are excerpts:
“We have identified three documents falling under the scope of your request (hereinafter the ‘requested documents’), namely:
– 6 month report on clinical trials status of the TETRA project
– 12 month report on clinical trials status of the TETRA project
– 18 month report on clinical trials status of the TETRA project
Please note that the Commission is not in possession yet of the periodic report, which is expected to be submitted within the coming weeks by the Consortium of the TETRA project.
2. Examination under regulation (EC) No 1049/2001
Having examined the requested documents under the provisions of Regulation (EC)
1049/2001, and taking account of the legitimate interests of any third party concerned, we regret to inform you that access cannot be granted to the documents requested. Some information has been withheld, as it concerns the commercially sensitive information and the personal data, as explained below.
2.1 Protection of commercial interests of a natural or legal person, including
intellectual property Article 4(2), first indent, of Regulation (EC) 1049/2001 provides that “[t]he institutions shall refuse access to a document where disclosure would undermine the protection of […] commercial interests of a natural or legal person, including intellectual property, unless there is an overriding public interest in disclosure”.
The documents requested contain detailed information about the description of works and studies to be undertaken during the project, as well as a description of the specific project activities, milestones and the corresponding timetable of planned activities.
The public disclosure of this information might undermine the commercial interests of the participants to the TETRA project within the meaning of Article 4(2), first indent, of Regulation (EC) 1049/2001″.
and
“According to Article 4(1 )(b) of Regulation (EC) 1049/2001, access to documents is
refused where disclosure would undermine the protection of “privacy and the integrity of the individual, in particular in accordance with Community legislation regarding the protection of personal data”.
The requested documents contain personal data such as the name, surname, signature, email address, and function of some participants to the TETRA project and individuals external to the project. This information clearly constitutes personal data in the meaning of Article 2(a) of Regulation (EC) 45/20012″
I was at least informed that the patient recruitment is not happening yet and the ethics vote has not yet been applied for. But everything else seems to be almost in place:
“The ethics analysis and evaluation of the TETRA clinical trial will be done when the
TETRA clinical trial documents are prepared (Clinical trial approval – CTA submission to EMA and Ethics submission). However, the consortium keeps the Commission regularly up-dated with all information and developments concerning the preparatory work of the clinical trials with six-month status reports as obligatory and confidential project deliverables. These reports cannot be disclosed for the reasons explained above.
Besides the ethics assessment during proposal evaluation, the TETRA project has not yet undergone any other ethics review. Such a review has been foreseen by the Commission to take place before start of the actual TETRA clinical trial.
Until today, the TETRA consortium has submitted several deliverables to the European Commission independent from the submission of the periodic report. These deliverables contain technical information related to the project.
Up to now, only an advance payment of € 2,617,740.38 has been made to the coordinator of the project, in line with Article 21.2 of H2020 Grant Agreement. The coordinator must ensure that all payments are made to the other beneficiaries without unjustified delay.
Other information on the status of the TETRA project is confidential, as its disclosure
would undermine the implementation of the project and the commercial interests of the participants in the project”.
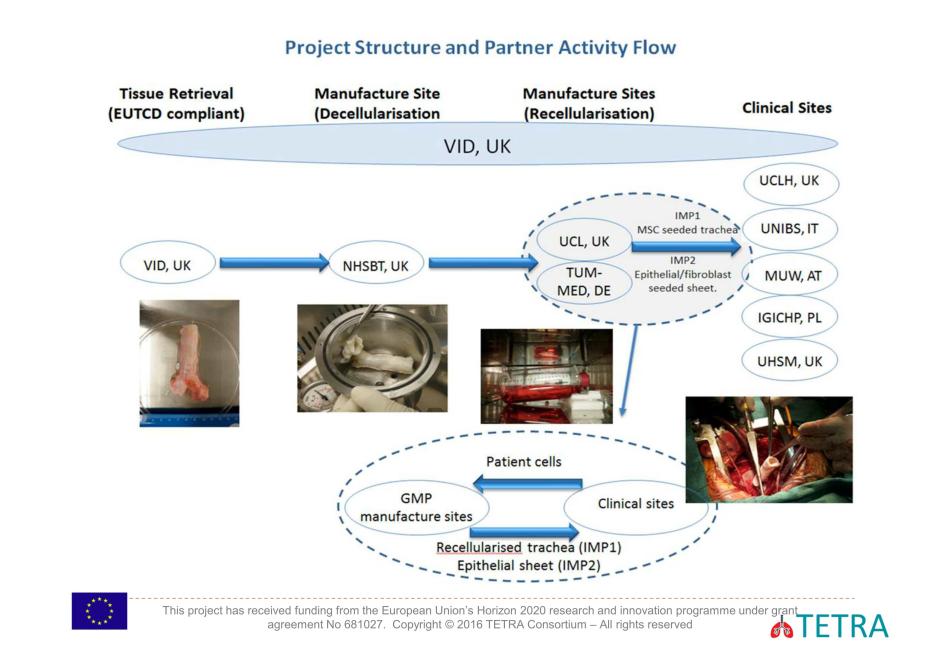
Full letter again, is here. The project coordinator is the Liverpool-based company Videregen, where Birchall is a paid advisory board member (read here). Nobody knows what Videregen exactly does, since they apparently don’t manufacture anything, and neither do they deal with any patients. But EU gives them the lion’s share of the funding money. Another key TETRA participant, Catapult, announced a huge investment in its Cell and Gene Therapy centre.

The actual science behind TETRA trachea transplant trial is mindbogglingly non-existent, outside of wishful thinking (see my article on this topic). There was one trachea transplant study with pigs, performed by Macchiarini and Birchall (Go et al 2010), which was heavily criticised for its poor science on PubPeer, just as Birchall’s other pig study was, which serves as justification for his similarly designed larynx transplant trial RegenVox. In May 2017, the British Health Research Authority informed me that that this trial, which uses basically same technology as INSPIRE and TETRA, but for larynx, has not recruited any patients yet.
But here Birchall himself explains what bone marrow cells allegedly do on his command:
“Having found themselves in certain environments a stem cell will look around and say ‘This has got the correct feel to it, the right stimuli, shape of a muscle, a piece of cartilage, a piece of bone’ and will therefore differentiate in those ways. So, for example, if it’s an environment with lots of other cartilage cells it’ll become a cartilage cell too”
So the scientific thinking behind TETRA is: the patient’s bone marrow cells seeded inside a bioreactor on a dead decellurised trachea carcass of a donor (there are NO cells to guide them, that is the whole point of decellurisation) will literally look around and say to each other: seems we are on top of a trachea-shaped thingy, so let’s make trachea cartilage! When this contraption is sutured into a patient, with hers or his own windpipe trashed into garbage, the endothelial cells on this patient’s blood vessels will scream: look, there is something rotting and trachea-shaped, with confused dying bone marrow and epithelial cells stuck to it, to the rescue! And these blood vessels will grow with a never before-heard supernatural speed, driven by the desire to save their little friends on Birchall’s masterpiece. No, I am not making it up. TETRA scientists do, and EU bureaucrats get all sweaty and hot over this fantasy.

Hence, not even Brexit can kill EU’s desire to help the British surgeon Birchall transplant some tracheas. In fact, Brexit might even prove beneficial, as the liberated Britain intends to relax or maybe simply abolish all patient protection and offer their own citizens to pharma and biotech industry for medical experiments (again, I am not really exaggerating, read my updated blog on this topic). TETRA’s main trachea transplant facility is to be under Birchall’s control, at UCL and its hospital UCLH, an additional UK trial site is designated with the University Hospital of South Manchester. Trachea transplant grafts will be manufactured in London and also in Munich, at the Technische Universität and its TUMCells centre (see this article). In fact this German state of Bavaria already had its own trachea transplant trial, led by former Macchiarini collaborators Heike and Thorsten Walles. The trial ran out without achieving a single milestone and never even recruited animals, never mind humans, for its experiments (read here).
Further TETRA participants, Università degli studi di Brescia (Italy), Medical University of Vienna (Austria) and Instytut Gruzlicy I Chorob Pluc (Poland), are scheduled as additional trial sites. And this is where the opportunity lies, in my opinion.
TETRA’s schedule is to start mass-transplanting their 48 patients by the next summer, as per my past communication with EU spokesperson from exactly one year ago:
“Ethics checks will also take place before the start of the planned clinical trial and mid-term. The ethics review is foreseen before the TETRA patient recruitment, which is scheduled to begin in July 2018”
It is likely that by that time the entire Macchiarini affair will be fully forgotten, certainly outside of UK, and all his and Birchall’s trachea transplant patients will quietly disappear into oblivion forever. Their corpses will finally stop blocking the progress of science. Because all TETRA documents are secret, Birchall and his partners would be able to write whatever they want without much danger of being found out (as they did before with INSPIRE, see here), and parade those dead and mutilated patients around as past success stories. On the basis of such alternative facts, a phase 2 trial might indeed obtain a positive ethics vote and commence without any phase 1 trial ever happening.
But even if EU should eventually get cold feet and shy away from approving this EU-wide mass trachea transplant orgy, patients will still likely get operated. With so much expensive equipment and technology in place, it would be a shame not to use it, especially if the TETRA progress report needs some clinical success to showcase. This is where the glorious tool of hospital exemption comes into place. In fact, all patients of Macchiarini’s and Birchall’s were operated as compassionate use cases, under hospital exemptions. And here, all rules and regulations are lifted, if you pick the right hospital. The regulation-free brexited UK and Macchiarini-ignorant Austria and Poland might prove the right place to go, even Italian authorities seem not to care much, despite Macchiarini’s horrendous patient abuse at Careggi Hospital in Florence.
All you need for your hospital exemption is patient’s signed consent. It can be just a piece of paper saying “yeah, whatever”, because no outsider will ever get to see it (certainly not in countries lacking a robust FOI law, like Sweden has and to a degree, also UK). The patient doesn’t have to be dying or even in any immediate danger to life, your educated guess as doctor suffices that a cadaveric trachea transplant is the best therapy option. You need no ethics vote whatsoever for hospital exemptions, as the above mentioned trachea transplanters Walles successfully proved, in Germany (read here).
Will the TETRA participants go down that road, if they unexpectedly fail to obtain the ethics vote for a proper clinical trial? Who knows. But if they do, EU bureaucrats who dream with sweaty palms of a European profit-raking trachea transplant industry servicing thousands of patients worldwide, will instead have blood on their hands.

These are the trachea transplant patients of Macchiarini and Birchall, on whose suffering TETRA is built (see the original list, with links to my relevant articles, here). Let us not forget these human beings.
- Claudia Castillo, born 1977, suffered from tuberculosis-damaged airways. Operated on 12.06.2008 at Hospital Clinic Barcelona, Spain. She received a cadaveric trachea graft which was implanted as a bronchus transplant, with the use of bioreactor for the purpose of regeneration. The organ was prepared without the knowledge of authorities in the veterinary lab of Martin Birchall at the University of Bristol. Claudia’s real medical state was very serious at all times, she lost her left lung last year.
- D.D., female, born 1953. Operated by Macchiarini on her airways twice before she was transplanted in October 2009 at Institut Dexeus in Barcelona, after the Hospital Clinic denied Macchiarini permission. This patient received a cadaveric trachea, which was smuggled from the Hospital Clinic. The patient was afterwards emergency treated 13 times in thoracic surgery because of dyspnoea (difficulties of breathing) caused by collapse of the graft, “without final resolution”. The graft detached soon after the transplantation, causing infection, tracheostoma was opened to save this patient’s life. Her current status is unknown.
- Ciaran Lynch, born 2000 without functional trachea, received a homograft from Martin Elliott which functioned for 10 years. The boy received on 15.03.2010 from Macchiarini a trachea transplant (prepared in a lab at Royal Free Hospital, London, provenance was later on officially assigned to Italy) at Great Ormond Street Hospital in London. It was a cadaveric trachea in full, regenerated by bionic method (no bioreactor). Martin Elliott, but also Martin Birchall, were involved in the operation.
- M.K., female, born 1979, from Czech Republic, at the time mother of a 7 month old baby. She had mucoepidermoid carcinoma, was delegated to Macchiarini by her Czech doctor, the thorax surgeon Václav Jedlička. Operated at Careggi Hospital, Florence on 06.07.2010. She received a cadaveric trachea, using bionic method (no bioreactor). She also received chemotherapy in parallel. Sent home with mediastenitis (inflammation of chest cavity) on July 30th, later on fistula formation, cancer recurrence with the necessity of repeated bronchological interventions and chemotherapy was diagnosed. The patient died 1 year after diagnosis (which means already in 2010 or early 2011).
- Keziah Shorten, born 1991, from UK. She had adenoid cystic carcinoma and was operated on 13.07.2010 at Careggi, Florence, with a cadaveric trachea in full, regenerated by bionic method (no bioreactor). She also received intraoperative radiation therapy (IORT). The therapy was according to my information managed by Birchall; Macchiarini’s partner in the Careggi operation theatre was UCL surgeon Paul O’Flynn. After the transplant failed, Keziah received from Martin Birchall a second transplant on 29.09.2011, Macchiarini was not involved. It was a plastic trachea, made at UCL and regenerated at Royal Free using a bioreactor. Keziah died in January 2012.
- G.M, female, born 1987, diagnosed with left-side bronchomalacia (softness of bronchi). She was operated on 28.09.2010 at Careggi, Florence and received a cadaveric trachea graft to replace her left bronchus. As severe complications with the graft ensued 2 months after the operation (aorto-bronchial fistula), she had her left lung removed and suffered “serious permanent brain damage”. Between 2010 and 2011 she underwent 7 interventions after transplantation. Current status unknown.
- M.M, female, born 1945, suffered from tracheo-oesophageal fistula, which happened postoperatively after larynx cancer treatment. She was operated on 04.10.2010 at Careggi, Florence with a cadaveric trachea, namely the cervical and media-trachea section. The patient suffered then from venal thromobosis and infection of tracheostomy, transplant became necrotic and she died around one year after hospital discharge in September 2012, “of sudden massive upper gastrointestinal bleeding”.
- Zhadyra Iglikova, born 1984. Russian patient who suffered tracheostomy after a car accident. She was operated by Macchiarini on 07.12.2010 at National Research Center of Surgery in Moscow and received a cadaveric trachea graft, namely cervical and mediastenic trachea section. She received “a tracheoplasty with a T-tube” in April 2011, which suggests that the cadaveric graft was removed. An article by Dzemeshkevich 2015 described “graft’s stenosis due to scarring and granulation tissue formation without signs of preservation of native tracheal structure and function”. Patient alive, otherwise current status unknown.
- F.F., male, born 1938, diagnosed with squamous neoplasia of trachea. Operated on 25.01.2011 at Careggi, Florence, received a cadaveric trachea graft, in full, with neocarena. The patient died very soon after, on 22.02.2011, after “massive pulmonary embolism”.
- Shauna Davison, female, born 1997. The 15 year old girl received a cadaveric trachea transplant from Birchall’s partner Martin Elliott, on 15th February 2012. Shauna died on March 9th 2012, according to her obituary she suffocated when her new trachea collapsed.
Update 5.01.2018. With a letter from 3.01.2018, EU Commission Secretary General Alexander Italianer rejected my request for information again, citing privacy and business interests of the trachea transplanters. He actually fears competitors would steal Birchall’s trachea transplant technology and build a successful industry with it. Also, he declares that large parts of TETRA documents deal with the phase 1 clinical trial Inspire, and as such outside of my right to request, even if it was my reporting which got Inspire grounded in the first place. This argument of Italianer I think this is the real reason why EU refuses access to TETRA documents:
“considering the sensitive nature of information in the documents, their public
disclosure could also cause reputational damage to both (partners of the) TETRA
consortium and the individuals linked with it”.
Regarding my request to consider the long list of patients who suffered and died using exactly same technology and release the documents to protect European patients, Italianer remains unconvinced and refers to these victims only once as “the claims you have made in your online article“. He concludes:
“In your confirmatory application, you do not put forward any reasoning pointing to an overriding public interest in disclosing the documents requested. Nor have I been able to identify any public interest capable of overriding the interests protected by Article 4(2), first indent, of Regulation 1049/2001”.
Update 9.01.2018. This position statement submitted by TETRA participant Catapult UK to the British Parliament indicates that Italianer and the EU Commission hid the existence of crucial TETRA approval documents from my FOIA request. When I asked for “the outcome of ethics reviews and the evaluation reports of all other evaluations the TETRA project experienced” I was informed that none would exist and only 3 progress reports are available which cannot be shared. However, European Medicines Agency (EMA) and EU Commission already greenlighted the use of cadaveric trachea for EU patients:
“A positive opinion on EU orphan designation for the treatment of tracheal stenosis was given by the Committee for Orphan Medicinal Products (COMP) (application number EMA/OD/069/16) and then adopted by the European Commission on 29th October 2017 (application number EU/3/16/1717).The product was classified as a Tissue Engineered ATMP according to Article 17 of Regulation (EC) 1394/2007 by EMA’s Committee for Advanced Therapies (CAT) on 9th November 2015 (Doc. Ref. EMA/CAT/650083/2015). However, the EMA have subsequently recommended a reclassification to a “combined ATMP” and this will be addressed in due course”.
Donate!
If you are interested to support my work, you can leave here a small tip of $5. Or several of small tips, just increase the amount as you like (2x=€10; 5x=€25). Your generous patronage of my journalism will be most appreciated!
€5.00

“patient’s bone marrow cells seeded inside a bioreactor on a dead decellurised trachea carcass of a donor will literally look around and say to each other: seems we are on top of a trachea-shaped thingy, so let’s make trachea cartilage!”
Or, these cells will look around, find dead trachea and say to each other- time to die….. That is what they obviously did when the method was tested on real humans.
The whole story looks like a horror movie…..
LikeLike
Perhaps this trachea transplant would have a probability of sucess and of becoming an useful tool….but with so many unsucceful stories what went wrong? Was the technique enough tested in animals? Where all the legal and safety procedures accomplished? The high rate of deaths would deserve stopping immediately any ongoing trials and an investigation in depth
LikeLike
Pingback: Alexander Seifalian, UCL’s Persian Scapegoat – For Better Science
Pingback: Martin Birchall’s shaky road to mass trachea transplanting – For Better Science
Pingback: Tissue-engineered tracheas: an assessment of the scientific, clinical and ethical implications – For Better Science
Pingback: Appeal to Italian Parliament for an investigation into trachea transplants in Florence by Paolo Macchiarini – For Better Science
Pingback: Image reuse, the new low of UCL trachea transplanter Martin Birchall? – For Better Science
Pingback: Indestructible Sumitran-Holgersson: Commit misconduct on patients, get EU funding to continue – For Better Science
Pingback: Trachea transplanters: Round 2 at UK Parliament – For Better Science
Pingback: UCL’s decellurised tracheas: strong and stable? – For Better Science
Pingback: EU trachea transplant clinical trial TETRA “uncertain to take place” – For Better Science
Pingback: Trachea transplanters without borders – For Better Science