The European Union Commission staunchly refuses to tell me what exactly their funded scientists intend to do to the scheduled 48 trachea transplant patients under the Horizon 2020-financed phase 2 clinical trial TETRA. By now the highest authority, the European Ombudsman, is engaged, and still EU Commission does not bulge. The general technology of TETRA and its indefinitely suspended phase 1 UK predecessor Inspire is however known: cadaveric tracheas from dead donors will be collected, decellurised to remove all the host cellular tissue, and then subjected to the magic of recellurisation in bioreactors, where bone marrow and epithelial cells will turn a dead carcass into a living organ, ready for transplant.
The cadaveric grafts must either to be obtained very fresh (a nightmare of impracticability), or kept frozen before they are needed, otherwise they will rot. Leanne Partington, a PhD student of the UCL trachea makers Mark Lowdell and Martin Birchall, investigated in her 2014 PhD thesis the effect of this freeze-thaw step on the decellurisation process (it proved to increase efficiency) but also on the graft stability, as measured by compression tests. This is where it turned out that the defrosted grafts lost roughly half of their mechanical stability. Which means they would collapse immediately when implanted into patient, which was indeed exactly what happened. Yet UCL and EU Commission want to keep trying, and according to the patented technology by the trial sponsor Videregen, defrosted tracheas are to be used. Which by EU business-oriented logic suggests, the patented technology from the freezer is to be used in TETRA.
The two experiments UCL performed on the patients Ciaran Lynch and Shauna Davison in 2010 and 2012, respectively (read here), used defrosted tracheas. Both trachea grafts collapsed right away, only that Birchall and his partners decided to omit this critical information about Ciaran’s trachea in their Elliott et al Lancet 2012 paper, while telling untruths about Shauna’s fate. The UCL trachea transplanters until very recently either forgot about Shauna’s existence, or pretended her new trachea functioned great up to her allegedly unrelated death just two weeks after the transplant.

The UCL PhD student Partington, who now works with the Inspire and TETRA partner Cell and Gene Therapy Catapult, had a difficult start. She had to first establish the special decellurisation technology of the great master Paolo Macchiarini in Birchall’s and Lowdell’s UCL lab. Nine patients were treated by Macchiarini’s team with such grafts between 2008 and 2011 (read here and here). Most are dead, only Ciaran Lynch and two more survivors (whose grafts were since removed) are known to be still alive, while I was sued in court in Berlin by Macchiarini’s acolyte Philipp Jungebluth and am presently awaiting my appeal hearing, for the same issue. What one can safely assume, is that Macchiarini did not use fresh tracheas for decellurisation, as suggested by the case of this British patient of UCL, where the Italian surgeon apparently had the grafts ready in advance. That patient, and another young woman operated just 2 days earlier, are both dead. This section in the thesis discussion describes Partington’s peculiar experience with Macchiarini’s team:
“The published methodologies of the original DEM [detergent-enzymatic method, -LS] (Conconi, De Coppi et al. 2005, Macchiarini, Jungebluth et al. 2008) and revised DEM (Jungebluth, Go et al. 2009, Go, Jungebluth et al. 2010) were ambiguous and despite personal communication with one of the published papers’ authors to obtain clarity on the DEM (Jungebluth 2009), misinterpretation of the DEM occurred and resulted in an adapted DEM being used in this thesis. In a later paper published in 2012, Jungebluth described the process more clearly stating that a single treatment cycle involved a 48 hour water incubation step followed by cycles of a 4 hour detergent step and a 3 hour enzymatic step (Jungebluth, Bader et al. 2012). Unless the authors were operating the process on a 24 hour basis, there was a 16 hour overnight incubation/holding step that was not described nor included in the published methods. The adapted DEM method described here (Partington, Mordan et al. 2013) however, was very similar to a further revised DEM method published by Baiguera and colleagues in 2010 (Baiguera, Jungebluth et al. 2010), with overnight PBS hold/wash steps”.

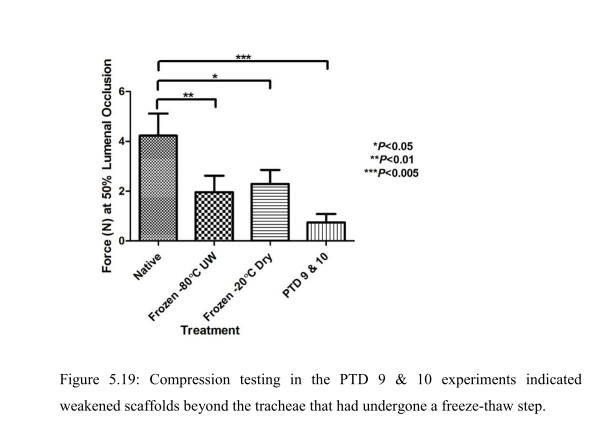

“The inability to store decellularised tracheal scaffolds without deterioration of the scaffold is a known issue (Baiguera, Del Gaudio et al. 2012). Therefore the use of a freeze-thaw step prior to decellularisation to aid both the decellularisation of the trachea and add flexibility to the commencement of the decellularisation process, by allowing storage of the trachea for up to one month was considered favourably. The step had been introduced to the concept of PTD [pressurised transmural
decellularisation, -LS] decellularisation after recommendation of the use of the freeze-thaw step from the decellularisation team at the Department of Surgical Research at Northwick Park Institute for Medical Research (NPIMR). Although the freeze-thaw step was clearly beneficial from a flexibility point of view and could be aiding the decellularisation, it was clear from the compression testing results that the freeze-thaw step was detrimental and caused a 50% reduction to the biomechanical strength of the scaffold. It was therefore decided to investigate if the PTD decellularisation could be successfully performed on fresh trachea that had not undergone any freeze-thaw steps”.
“The final PTD decellularisation process described in this thesis using fresh (non-freeze-thaw treated) tracheae had only been performed using one condition after it was realised that using fresh trachea was a critical criteria for obtaining a product that could potentially attain the assumed critical quality” attributes of the decellularised scaffold product.

In fact, while Partington was testing various protocols for her PhD thesis and finding decellurised trachea to be rather floppy, two patients were operated at UCL with such trachea transplants. Also here the published outcomes deviate from what was really observed.
According to the Lancet paper Elliott et al 2012, describing Ciaran’s success story, the trachea graft that boy received in 2010 performed without any major problems, even the stent (which was inserted by Macchiarini and Martin Elliott already pre-implanation) seemed unnecessary:
“6 weeks after surgery the stent had dissolved and there was mild collapse of the proximal graft. A shorter (10×45 mm) PDO stent was implanted under fluoroscopy. The patient underwent bronchoscopy or balloon dilatation under fluoroscopy, or both, regularly for 6 months (figure 2B). The major reason for further bronchoscopy or balloon dilation was mucus retention and crusting within the native bronchi in which there were still embedded metal stents. At 5 months, after dissolution of the latest stent, we remained concerned about the rigidity of the proximal graft, and so overlapping, self-expanding Nitinol stents (S.M.A.R.T. Control, Cordis, Waterloo, Belgium) were implanted into the trachea. At 6 months after the initial surgery the graft seemed stable, the patient’s airway was patent, and he returned to school.”
Vondrys D, Elliott MJ, McLaren CA, Noctor C, Roebuck DJ.
First experience with biodegradable airway stents in children.
Ann Thorac Surg. 2011 Nov;92(5):1870-4. doi: 10.1016/j.athoracsur.2011.07.042.
Ciaran is most obviously Patient 4 in that paper, and there, a different, more honest story is told. Ciaran’s graft collapsed, and could only be kept open, with great difficulties, by stenting. One wonders, if the authors admitted this, would Lancet have published their paper the next year?
“This patient, with an original neonatal diagnosis of long segment tracheal stenosis treated by pericardial patch tracheoplasty, followed a year later by a tracheal homograft transplant and subsequent stenting presented after a further 10 years with hemoptysis, caused by erosion of a balloon-expandable metal stent into his aorta. This was treated by stem cell–supported transplantation of the trachea and repair of the aorta. A polydioxanone stent was used during this operation to support the donor graft. The stent progressively lost its color and strength, and dissolved completely at 17 weeks.
There was severe progressive stenosis of the neotrachea, which did not respond to repeated balloon dilation. After tracheal predilation to 12 mm, a 12 mm x 65 mm polydioxanone stent was deployed in the neotrachea, but only expanded to about 7 mm, and was therefore too long. Bronchoscopy showed that the upper end of the stent was in contact with the vocal cords, and it was removed.
A 10 mm x 60 mm polydioxanone stent was deployed 1 week later, followed by a fourth polydioxanone stent after a further 6 weeks. Despite this, there was progressive stenosis, and 3 months later, a self-expanding nitinol stent was deployed in the distal trachea, with a dramatic improvement in symptoms.”

This is now the exact technology which UCL and its EU partners intend to use on over 50 patients. In spring 2017, a 2 year-old child received such a UCL-made trachea transplant outside of clinical trial, as a compassionate use case. The case report is not published yet.
The main selling point of TETRA and Inspire is that their trachea stenosis patients will be spared conventional therapies of stent insertion. Yet Birchall and his partners know very well that decellurised tracheas collapse, even more so the defrosted ones. These “neotracheas” collapse always and rapidly, in every single patient, of whom many died. The UCL scientists could already expect this already from their in vitro lab tests.

Donate!
If you are interested to support my work, you can leave here a small tip of $5. Or several of small tips, just increase the amount as you like (2x=€10; 5x=€25). Your generous patronage of my journalism will be most appreciated!
€5.00


Pingback: Boletim de Notícias: R$ 32 milhões ficam congelados no Ministério do Meio Ambiente | Direto da Ciência
Around 9:46 in this 2014 talk Martin Elliott explained Ciaran needed bronchoscopy every 4 hours to keep the magic trachea open. Oddly this information is not in Lancet 2012
LikeLike
Pingback: EU trachea transplant clinical trial TETRA “uncertain to take place” – For Better Science
Pingback: Birchall’s two dead pigs to prove trachea transplants are safe – For Better Science
Pingback: Macchiarini victim’s family sues trachea makers for wrongful death – For Better Science
Pingback: Macchiarini victim Zhadyra Iglikova is dead – For Better Science
Pingback: UCL trachea transplants: Videregen sets lawyers on Liverpool academics Murray and Levy – For Better Science
Pingback: Paolo De Coppi and the UCL organ factory – For Better Science
Pingback: Macchiarini partner Anthony Hollander chairs mass-sacking committee in Liverpool – For Better Science