Bert Vogelstein is God. This Johns Hopkins University professor has cured cancer ages ago, so far only in mice, but not yet in humans. Those who also managed to cure cancer in mice in Vogelstein’s lab become big professors themselves. Like Kenneth Kinzler, who remained at Johns Hopkins, or Heiko Hermeking, who returned to Germany to become professor at the Ludwig Maximilian University (LMU) in Munich.
But some papers by Vogelstein, Kinzler and especially Hermeking’s were questioned on PubPeer. A harassment campaign? Or rogue collaborators and PhD students having been sloppy? Both?
Vogelstein and Kinzler never replied to my email inquiry. Hermeking told me that Vogelstein suspects to be behind those baseless comments his envious peers, whom he intends to take legal action against. And to me personally, Hermeking issued this warning:
“…if you post/publish unfounded, reputation-damaging claims, which hinder me and my colleagues in carrying out our research, there any ways to respond to this since yesterday“.
The reference in this context went obviously to an earlier German Supreme Court decision about hate-postings against politicians. So I went and tried to debunk the PubPeer allegations on Vogelstein’s and Hermeking’s papers. Here we go.
The evidence on PubPeer is mostly years old, starting 2015. But it all came up after Clare Francis reminded Nature’s editor Barbara Marte about an old paper by Vogelstein, Kinzler and Hermeking.
Timothy A. Chan, Heiko Hermeking, Christoph Lengauer , Kenneth W. Kinzler, Bert Vogelstein, 14-3-3Sigma is required to prevent mitotic catastrophe after DNA damage Nature (1999) doi: 10.1038/44188
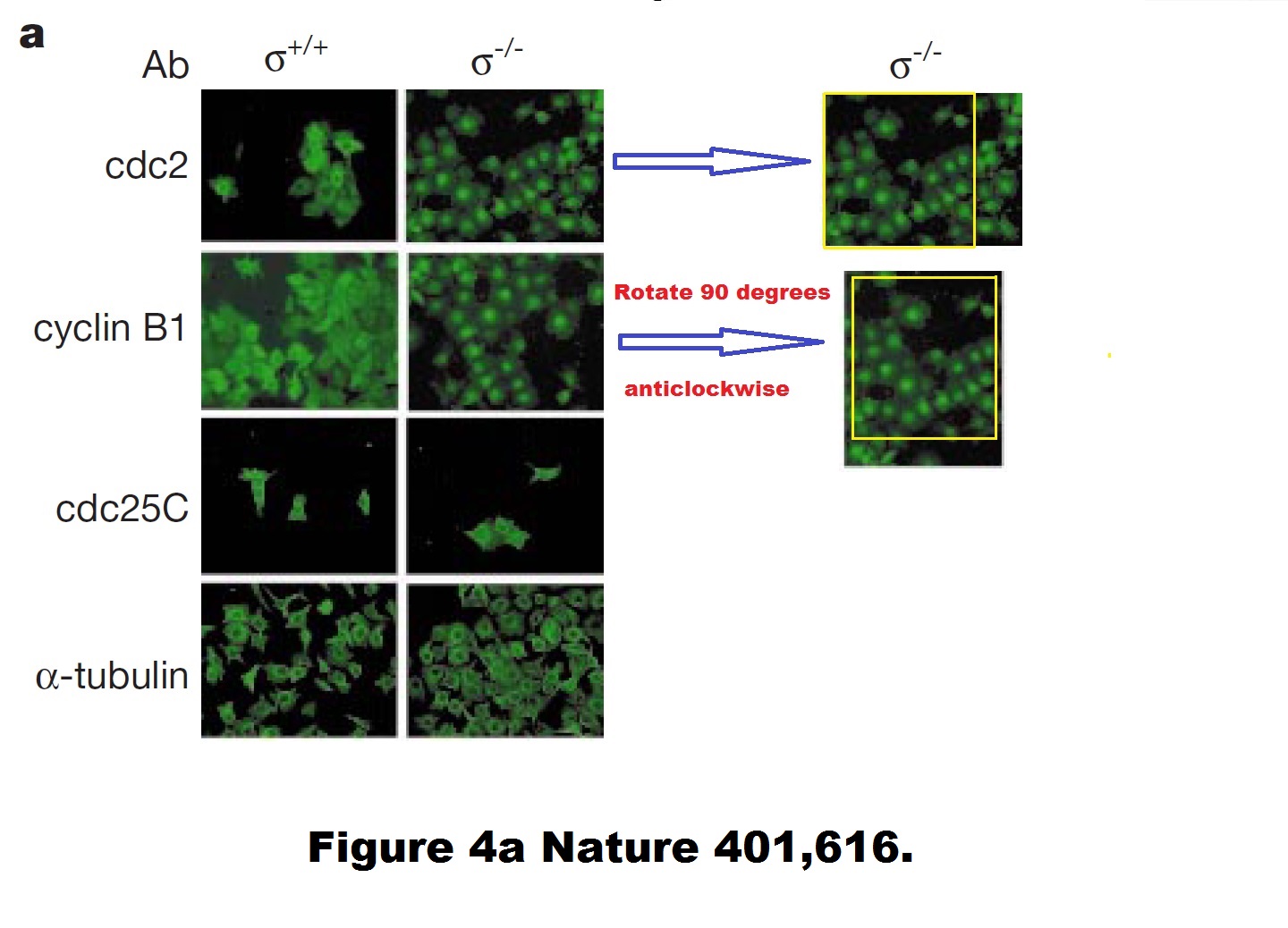
A duplicated and rotated image? Never! Hermeking explained to me:
“…as outlined in the responses to the Nature 1999 paper on PubPeer, CDC2 and cyclin B1 have the same staining pattern, i.e. they co-localize. Since green was always used as the color in the figure (with LSM-IF you get black and white pictures that are recolored), it looks as if the same image was used twice.
Unfortunately, the PhD student used the same image section of a double staining for CDC2 and cyclin B1 and rotated the photograph of the latter 90 degrees. He therefore did not use the same photo twice.“
That PhD student, Hermeking later explained, was Timothy Chan, now head of an oncology centre at Case Western Reserve University in Ohio.
But still, how can two different antibody stainings produce a virtually pixel-identical result? And even if such molecular biology miracle did happen, why are the cdc2/Cyclin B1 stainings different for +/+ cells? Hermeking was kind enough to teach me some basic cell biology:
“As you surely know, cyclin B1 and CDC2 bind directly to each other. Therefore, co-localization is to be expected.
As far as I remember, Mr. Chan was asked by Prof. Vogelstein to check this in the original images and he has confirmed that these are 2 different images. When staining the +/+14-3-3sigma cells, Mr. Chan selected two different sections.“
Such amazing 100% colocalisation of two antibodies for two different proteins, in immunofluorescence no less! Other people would never get an identical staining even when using two different Cyclin B1 antibodies. But then again, such failed scientists won’t ever succeed in Professor Vogelstein’s lab, like Professors Chan and Hermeking succeeded. Science is not for everyone.
There as another issue in that paper. Elisabeth Bik found something:
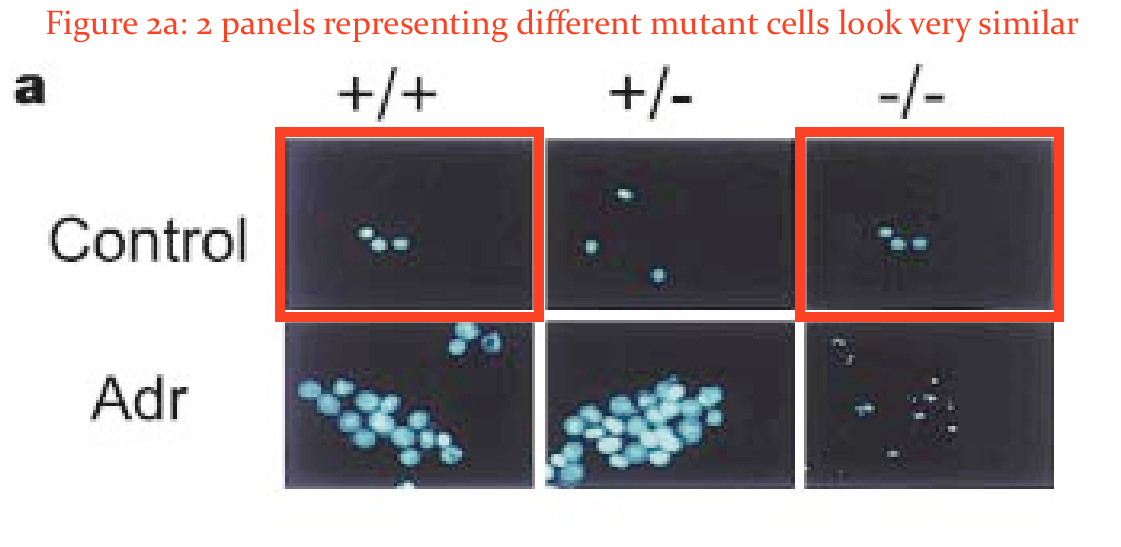
In addition, would the two panels in Figure 2A marked here with red boxes expected to look so similar?”
Hermeking was unimpressed by Bik’s amateurish and unqualified attempts to spot duplications:
“If you zoom a lot into Fig. 2A, you can see, in my opinion, that it’s not the are the same cell nuclei.
These are the results and figures by Timothy A. Chan.“
Regarding that 1999 Nature paper, Hermeking also declared to me:
“I would also like to note that since the comments are anonymous, the PubPeer Forum is unfortunately sometimes used to express personal opinions on the research of others that are not purely factual in nature. Whatever the motivation. This unobjective criticism is also expressed in the discussion of the Nature Paper from 1999 (Chan et al., position 2 in your list) and this is discussed/criticized at length by other participants (I myself have never made a contribution/ an answer in this forum because colleagues advised me against it). In my opinion, it is well known that not all studies on the same topic come to the same conclusion. In this case, an author with a publication on the function of 14-3-3sigma that had a different/different result appears to be using the PubPeer forum to discredit authors and their co-authors who disagree with him. This is the common conclusion which the authors of the Nature 1999 publication came to. The last author, Prof. Bert Vogelstein (Johns Hopkins Medical School Baltimore, USA), already proposed legal action against these comments a few years ago, as they are obviously not objective. So far we have refrained from doing so.“
Legal action? Against whom? Hermeking swiftly warned me that he will sue me for so-called hate-posting, see the quote above.
The pseudonymous Clare Francis, unaware of these threats, kept complaining to Nature. By mistake, the editor Marte hit reply-all instead of forward:
“Just what I needed before the weekend. So she (which I think is a he) is back. I think I handled this paper. 1999. I don’t believe at all that they had contacted us, because as you know I take these things seriously. They do look very much like duplications. PubPeer also refers to papers that couldn’t reproduce the findings. Oh lord…. I doubt it’s on ejp Vic and I will look into it. Barbara“
Marte later announced to Clare Francis and to me to investigate this paper. Good luck!
But Vogelstein doesn’t need Nature. He published two papers in Science which revolutionised cancer research, or so we are expected to think. The one from 7 years ago, Tomasetti & Vogelstein 2015, proclaimed the new wisdom on where cancer really comes from:
“….random errors occurring during DNA replication in normal stem cells are a major contributing factor in cancer development. Remarkably, this “bad luck” component explains a far greater number of cancers than do hereditary and environmental factors.”
The older you get, the more often your stem cells divide, thus the higher probability for cancerous mutation. A lot of scientists laughed at this weird and silly assumption, so to shut them up, Vogelstein issued a follow-up paper, also in Science, Tomasetti et al 2017, where he expanded on his theory, that two thirds of all cancers are indeed caused by bad luck of random genetic mutations only. Nobody will dare to argue with TWO Science papers!
Still, scientists were not prepared to accept the gospel because it was too silly. Both papers were rightly criticised by many peers, some of defunct PubMed Commons criticisms are preserved on PubPeer, here and here.
Now, I don’t know what exactly Vogelstein meant to convey. It seems he is keen to sell personal cancer-risk diagnostics, being involved with many biotech companies, as his conflict of interest statement reveals:
“B.V. is on the scientific advisory boards of Morphotek, Exelixis GP, and Sysmex Inostics, and is a founder of PapGene and Personal Genome Diagnostics. Morphotek, Sysmex Inostics, PapGene, and Personal Genome Diagnostics, as well as other companies, have licensed technologies from JHU on which B.V. is an inventor.”
But I am pretty sure certain other industries will also love Vogelstein’s teachings. See, cancer is actually not really caused by smoking, asbestos, radiation, environmental pollution, workplace hazards etc – it’s just bad luck. Science has spoken, twice.
Bad luck is also what befell some of Vogelstein’s papers. Like a duplicated flow cytometry plot here:
Paul M. Hwang , Fred Bunz , Jian Yu , Carlo Rago , Timothy A. Chan , Michael P. Murphy , Geoffry F. Kelso , Robin A. J. Smith , Kenneth W. Kinzler , Bert Vogelstein Ferredoxin reductase affects p53-dependent, 5-fluorouracil–induced apoptosis in colorectal cancer cells Nature Medicine (2001) doi: 10.1038/nm1001-1111
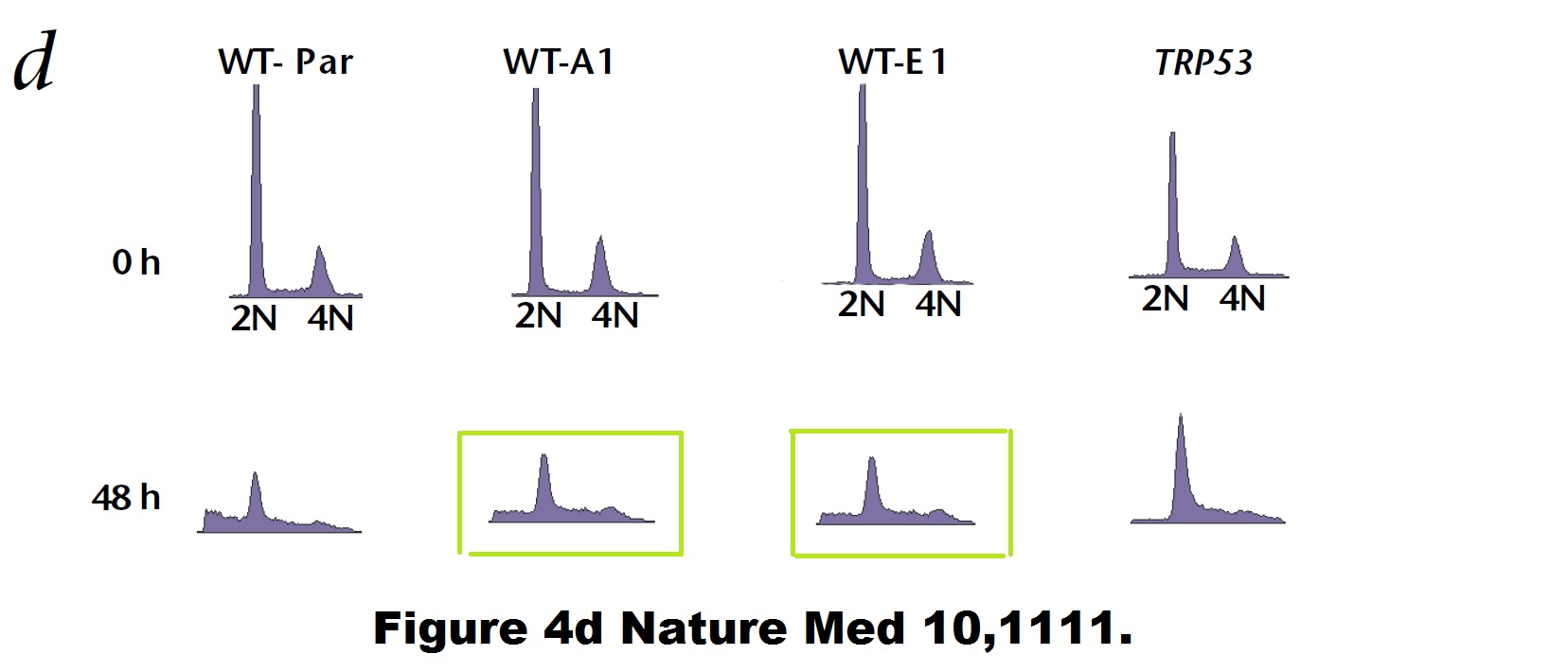
Bad luck was also what these mice had. Apparently, Vogelstein and Kinzler are such big stars of cancer research, that Johns Hopkins University allows them to do to their mice what pedestrian scientists are not allowed to do, due to concerns about animal welfare and cruelty.
B. H. Park , B. Vogelstein , K. W. Kinzler Genetic disruption of PPARdelta decreases the tumorigenicity of human colon cancer cells Proceedings of the National Academy of Sciences (2001) doi: 10.1073/pnas.051630998
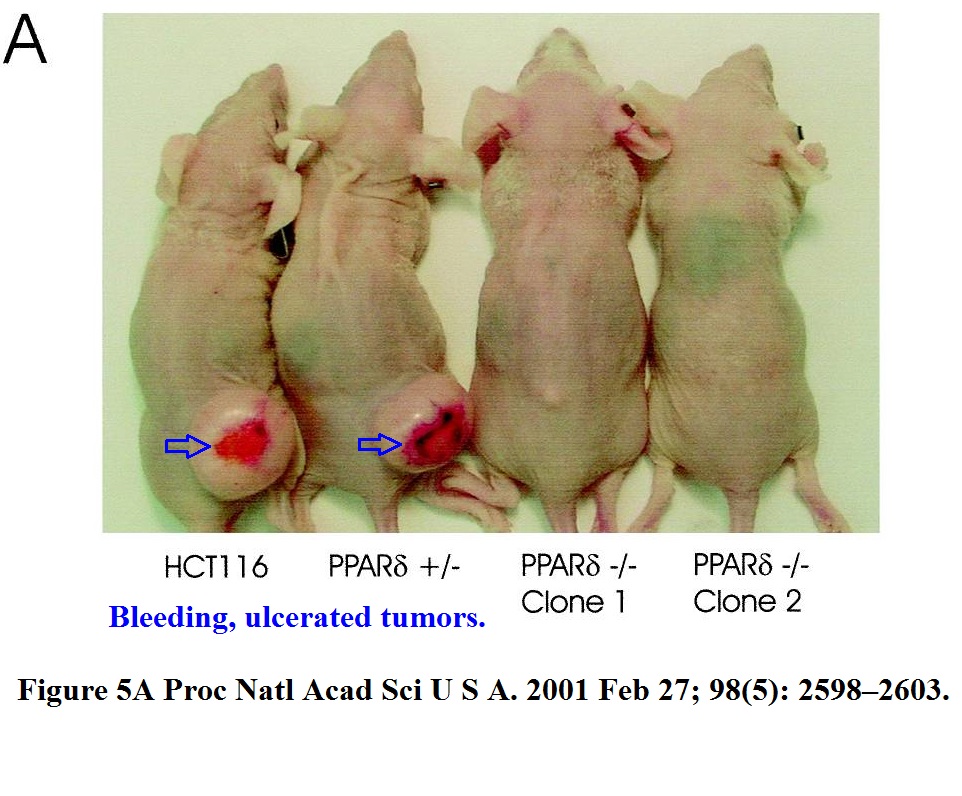
These immune-deficient nude mice suffered a lot, but it was not in vain. They were tortured for a paper which the National Academy of Sciences member Vogelstein “contributed” to PNAS. The study was namely so groundbreaking he chose his own peer reviewers, who apparently also agreed, that yes, the mice got what they deserved.
Let’s look at some strange gels instead.
Yardena Samuels , Luis A. Diaz , Oleg Schmidt-Kittler , Jordan M. Cummins , Laura Delong , Ian Cheong , Carlo Rago , David L. Huso , Christoph Lengauer , Kenneth W. Kinzler , Bert Vogelstein , Victor E. Velculescu Mutant PIK3CA promotes cell growth and invasion of human cancer cells Cancer Cell (2005) doi: 10.1016/j.ccr.2005.05.014
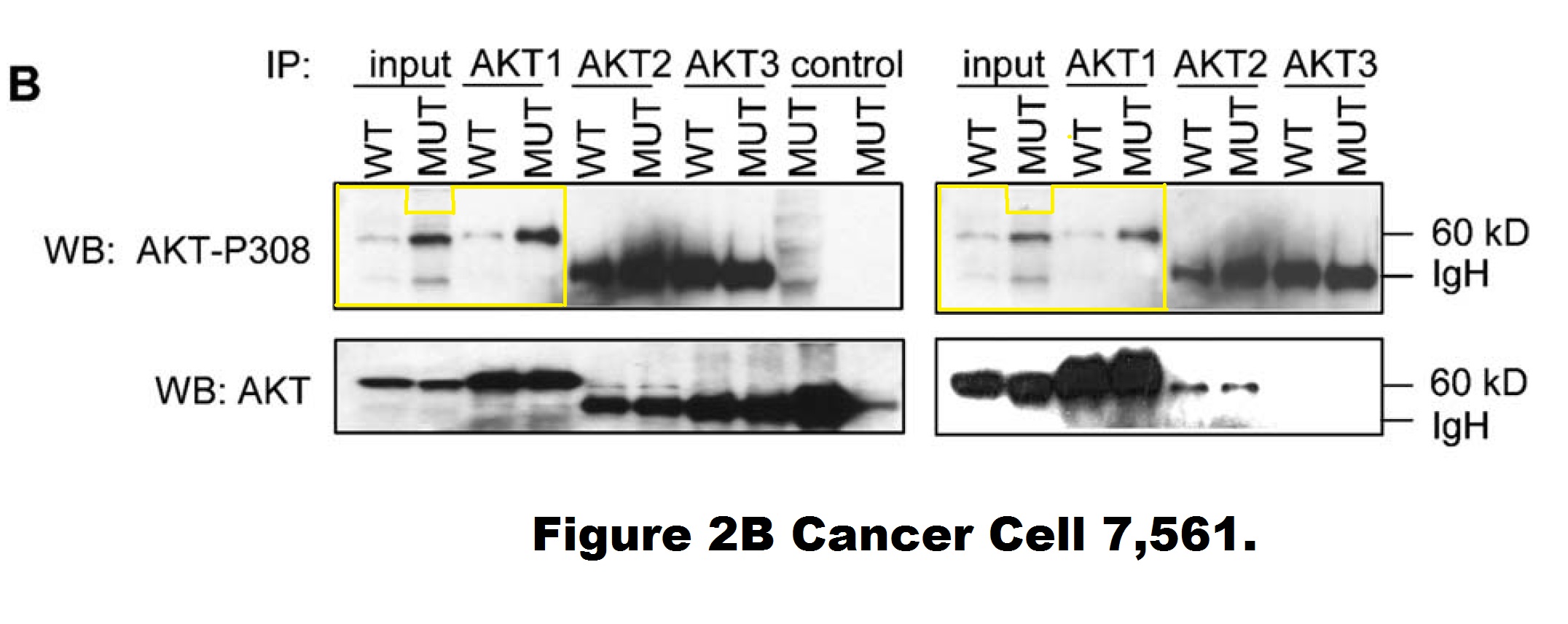
A closer look into this figure reveals that the entire right-hand phosho-AKT gel (showing DLD1 cells) is quite possibly a shorter exposure of the left-hand phosho-AKT gel (showing HCT116 cells). Those are likely two separate image acquisitions of the same blot (of unclear origin). It is difficult to explain as a mistake of oversight, since those are supposed to be different cell lines. Moreover, the HCT116 panel contains two extra two lanes (“control MUT”) on the right, the last one of which appears digitally spliced on. Vogelstein and his academic offspring at Johns Hopkins, the group leaders Kinzler and Velculescu did not reply to my email when asked to explain.
But Hermeking replied. He even provided me with a pdf addressing the PubPeer criticisms of his papers. Here is the file, I will quote from it below:
Once, Vogelstein, Hermeking and their colleagues even had to issue a correction over spliced gels. It was an interesting one, if one looks closely.
Yu-Wen Su , Zhenyue Hao , Atsushi Hirao , Kazuo Yamamoto , Wen-Jye Lin , Ashley Young , Gordon S Duncan , Hiroki Yoshida , Andrew Wakeham , Philipp A Lang , Kiichi Murakami , Heiko Hermeking , Bert Vogelstein , Pamela Ohashi , Tak W Mak 14-3-3sigma regulates B-cell homeostasis through stabilization of FOXO1 Proceedings of the National Academy of Sciences (2011) doi: 10.1073/pnas.1017729108
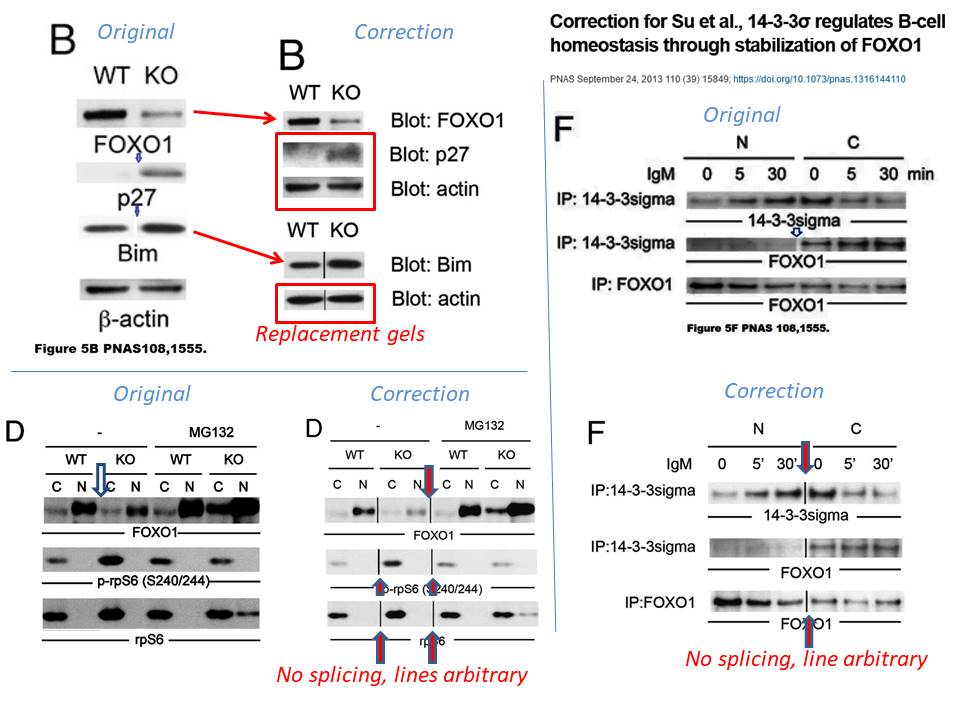
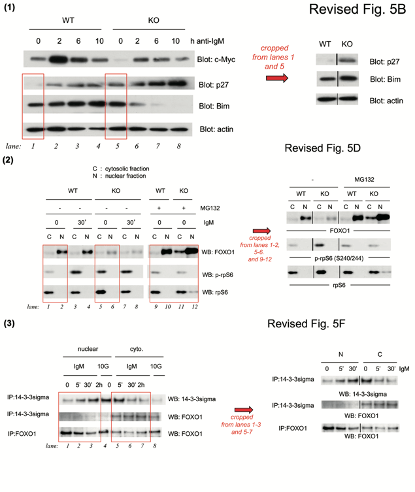
The correction’s text was not exactly honest. First of all, half of the data in Figure 5B was stealthily replaced, it is not clear why those old bands were removed and new results produced from nowhere. Well, it is clear, actually. The authors’ problem was: they could not just admit the irregular gel splicing, because it would render the entire figure meaningless and raise reasonable suspicions of data manipulation. In Figures 5D and 5F, the blots did remain same, but splicing lines were added. Yet not just where that splicing actually occurred, but also in other positions, where the gels were not spliced at all, if my own eyes are to be trusted. But with al these extra divider lines, the trickery looks more or less innocuous, and not at all like data fudging. Clever!
In any case, Hermeking states that these experiments were done by a “postdoc from the lab of Tal Mak in Canada“, i.e. the University of Toronto professor Tak Mak who also contributed this paper to PNAS as Academy member.
Update 10.02.2022. Now Tak Mak sent this postdoc to threaten me with a lawsuit also. This message just arrived, with Mak in cc, from Yuwen Su, now PI at National Health Research Institutes in Taiwan:
“My name is Yuwen Su, and I am the first author who performed the experiments of the PNAS paper you mentioned. First of all, I thank you for the great interest for reading the paper and for points out my correction. The experiments were done when I was a postdoc. fellow in Mak’s laboratory.
The corrected figures were repeatable with the same conclusion. The related original figures were sent to editor when we did the correction. To response your points: (1) The lower panel in the revised 5B was spliced from the same gel in one experiment from lanes 1 and 5 of the original gels. (2) The lines in revised 5D were cropped from lanes 1, 2, 5, 6, and 9 to 12. (3) The lines in revised 5F were cropped from lanes 1, 2, 3, 5, 6, and 7 of the original gels.
I would appreciate that if you withdraw the false allegations about the results. We reserve the right to take legal action, if you keep spread the false statement.“
The above low-resolution 128 kb image was attached. Not even of the full-size scans of original gels, but of a PowerPoint with dividing lines drawn and exported as a blurry image, which is pointless to scrutinise. The published Figure 5 and its correction have a much higher resolution. The paper was 2 years old when the correction was issued, is that all they have left of the raw data? And Su is confused, the revised figure 5B of the published correction is certainly not exactly what her picture shows, legal action or not.
Why do these scientists respond to reasonable criticisms of their works only with threats of lawsuits? Shouldn’t their science speak for itself? Didn’t Mak himself once accuse a colleague of “cheating”, and now he sends his first author to threaten me with legal action for failing to see in the original published figure the splicing lines where they drew them?
In yet another correction, it was also a collaborator. A problematic flow cytometry figure had to be corrected, which Hermeking states was done at the Cornell University, meaning in the lab of Alexander Nikitin.
Chieh-Yang Cheng , Chang-Il Hwang , David C. Corney , Andrea Flesken-Nikitin , Longchang Jiang , Gülfem Meryem Oner , Robert J. Munroe , John C. Schimenti , Heiko Hermeking , Alexander Yu. Nikitin miR-34 cooperates with p53 in suppression of prostate cancer by joint regulation of stem cell compartment Cell Reports (2014) doi: 10.1016/j.celrep.2014.02.023
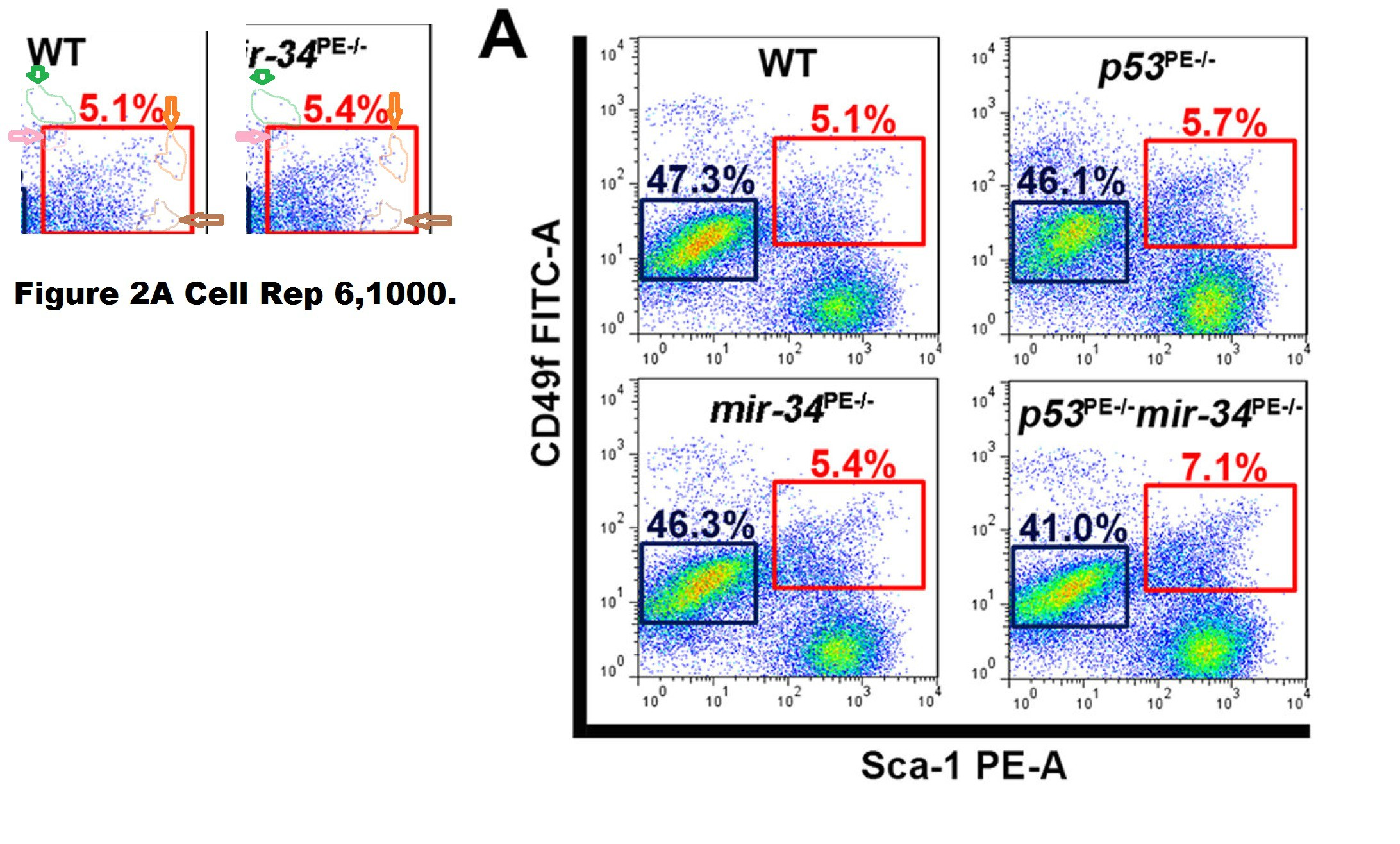
The Correction issued in 2015 declared:

“In Figure 2A of the originally published version of this paper, FACS plots for the wild-type and mir-34PE−/− samples were inadvertently switched during preparation. The corrected Figure 2A is provided below and now appears with the corrected version of the manuscript online. None of the conclusions in our manuscript are affected by this error.“
The “WT” FACS plot was replaced in the correction. Thing is, it was not the case that the mir-34PE−/− plot had been just accidentally copied on top of the WT one. The two FACS plots show the same measurement but plotted slightly differently, i.e., with slightly different gates which changes the overall appearance but retains much of the cell pattern. How can such things happen by mistake?
Nikitin protested that I should not believe my own lying eyes:
“All gate windows are the same in all images. All numbers were accurate in our original iterations of the figure. Unfortunately, during reformatting the figure for another journal, the correct WT image was inadvertently replaced with a wrong one, while the original, accurate numbers remained. During preparation of our erratum, all issues were discussed with journal editor and all documentation to substantiate our explanation was provided.
Your efforts to keep science accurate and reliable are certainly appreciated. However, it seems focusing on mistakes that directly impact data conclusions may better benefit research community.“
But I am trying, Dr Nikitin! Here, look, I wrote about your senior colleague, the Weill Cornell dean Augustine MK Choi:
But how does one know which mistakes affect the conclusions and which don’t? Maybe the authors should publish a disclaimer at the end of each paper which figures are important (if any) and which are just filler illustrations? Pictures with no other purpose but to lighten up the textual monotony of scholarly treatises?
Is the Figure 1b of this paper from Hermeking’s own lab in Munich just decoration or part of the conclusions?
Gerold Untergasser , Heike B Koch , Antje Menssen , Heiko Hermeking Characterization of epithelial senescence by serial analysis of gene expression: identification of genes potentially involved in prostate cancer Cancer Research (2002) issn: 0008-5472

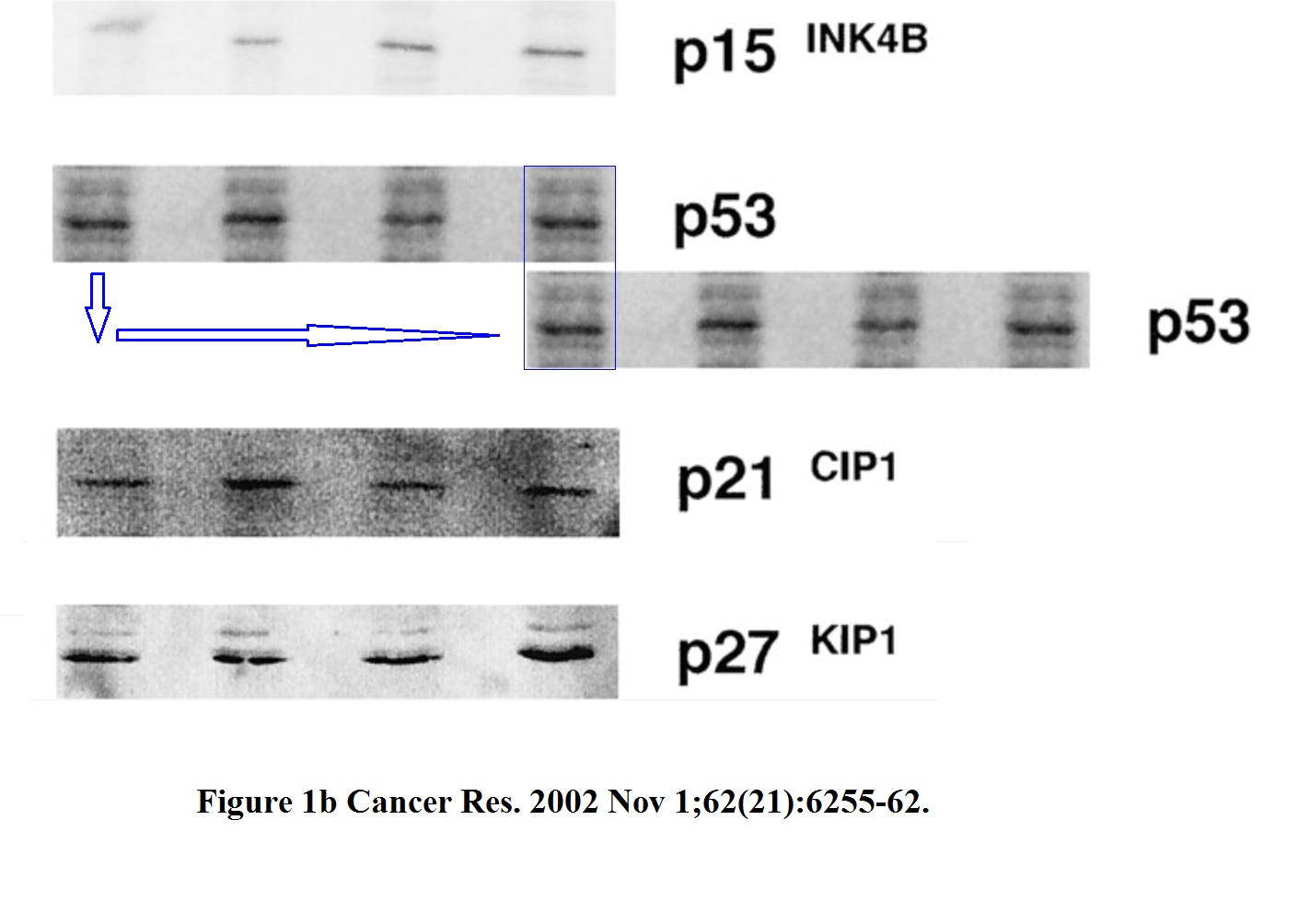
The two highlighted p53 bands (passage 5. and 8.) are too similar, such unexpected similarity in both the both the band shape and the background signal is highly unlikely to arise by chance. The minor pixel differences can be explained by image compression, while the overwhelming similarities cannot be explained at all.
But Hermeking disagrees:
“Gel bands similar, but not identical”
Hermeking declared exactly same for the following paper:
Dimitri Lodygin , Joachim Diebold , Heiko Hermeking Prostate cancer is characterized by epigenetic silencing of 14-3-3sigma expression Oncogene (2004) doi: 10.1038/sj.onc.1208004
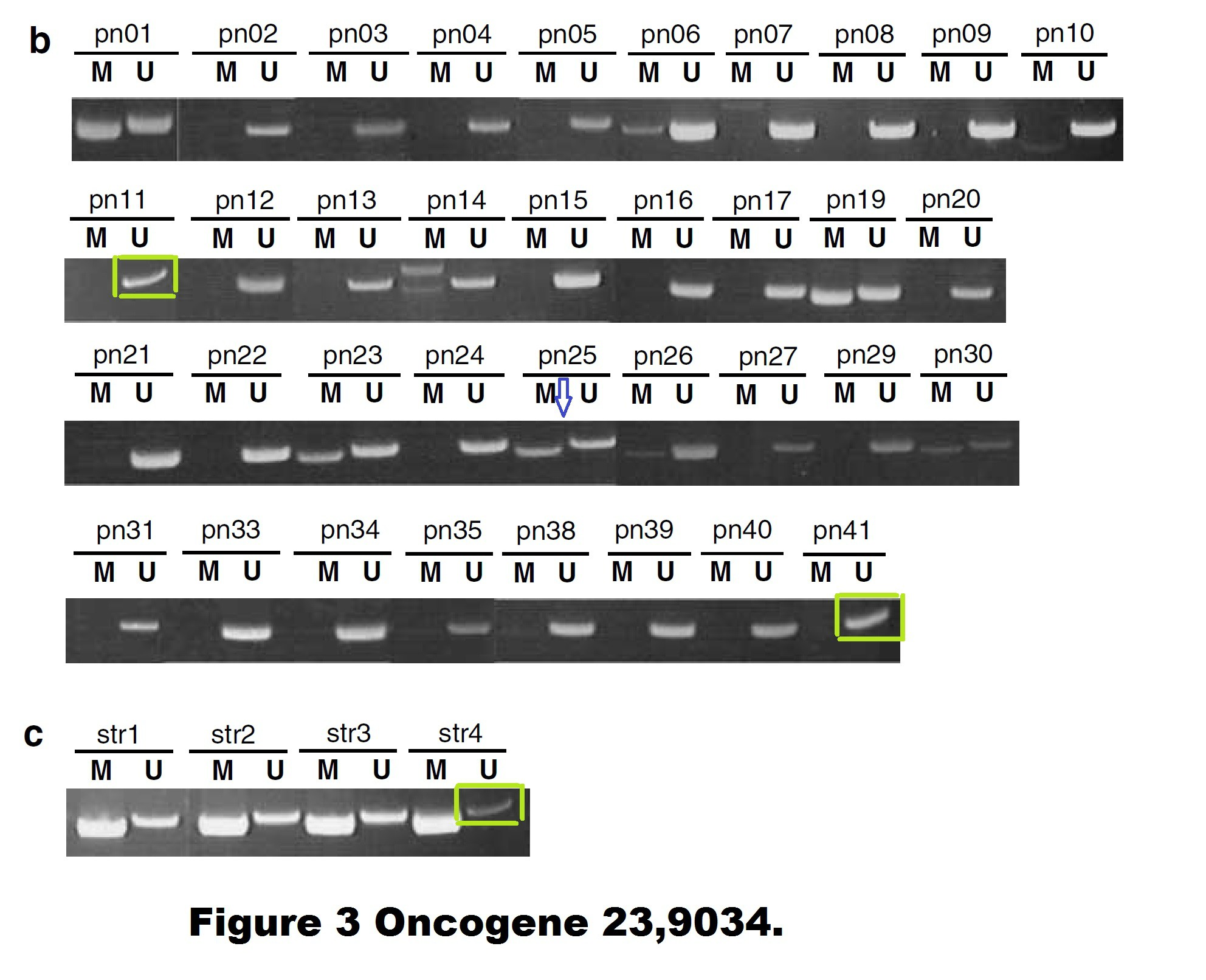
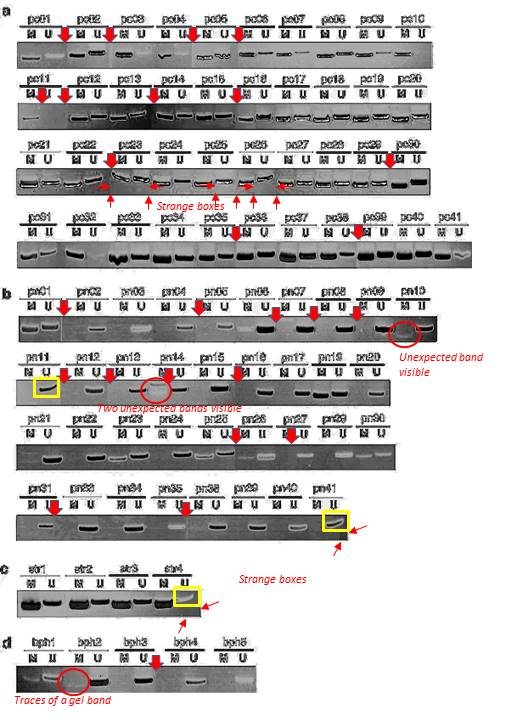
Quite possible indeed that these gel bands, flagged on PubPeer in 2015, are not identical. But the Figure 3 is a disaster in many other ways. The allegedly continuous gels are actually Franken-gels spliced from many pieces. There are strange gel bands of unexpected sizes lurking in the shadows, there seem to be strange box-shaped objects hoovering below some of the gel bands, how can all of that be explained?
I wrote to Hermeking and the first author Dimitri Lodygin, now group leader at the University of Göttingen in Germany. They remained silent.
There are also other issues with that paper. A PubPeer commenter insisted in February 2020 that some gels might be too similar to the gels in another study from the Hermeking lab. It is difficult to check, the resolution of the gels is extremely low, maybe Lodygin and Hermeking will find at least the original figure files they submitted to the journal?
Dimitri Lodygin , Alexey Epanchintsev , Antje Menssen , Joachim Diebold , Heiko Hermeking Functional epigenomics identifies genes frequently silenced in prostate cancer Cancer Research (2005) doi: 10.1158/0008-5472.can-04-4407

There is also this, flagged already in 2015, according to Hermeking the bands are again “similar, but not identical“:
These SFRP1 bands do look quite unexpectedly similar though. And the rest of the figure is a bit strange, too. Again those strange box-shaped shadows lacking all background, difficult to explain with image compression alone… In one case, a RASSf1A band looks as if it is inside a box.
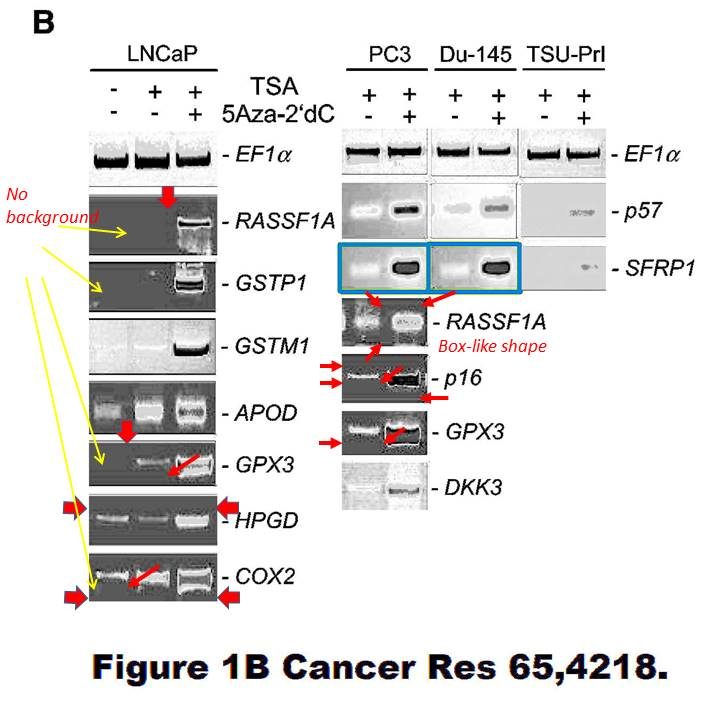
Again, maybe the authors have higher resolution versions of those gel images? Maybe the authors should check all their gel figures?
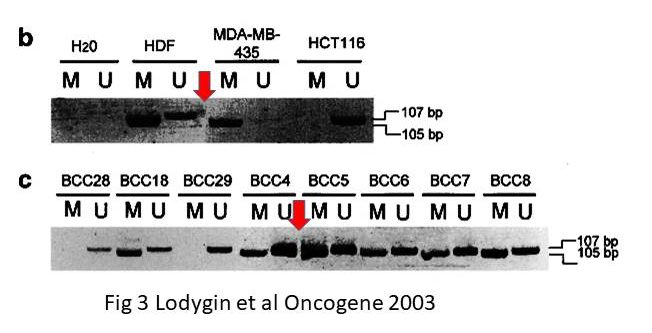
Dimitri Lodygin , Amir S Yazdi , Christian A Sander , Thomas Herzinger , Heiko Hermeking Analysis of 14-3-3sigma expression in hyperproliferative skin diseases reveals selective loss associated with CpG-methylation in basal cell carcinoma Oncogene (2003) doi: 10.1038/sj.onc.1206854
However, it seems that in the only gel figure of that paper, there is some possible splicing. If true, it was extremely difficult to find.
In an old collaborative paper, where Hermeking is one of the middle authors, a PubPeer user alleged in 2015 some gel band duplications:
A. T. Ferguson , E. Evron , C. B. Umbricht , T. K. Pandita , T. A. Chan , H. Hermeking , J. R. Marks , A. R. Lambers , P. A. Futreal , M. R. Stampfer , S. Sukumar High frequency of hypermethylation at the 14-3-3 sigma locus leads to gene silencing in breast cancer Proceedings of the National Academy of Sciences (2000) doi: 10.1073/pnas.100566997
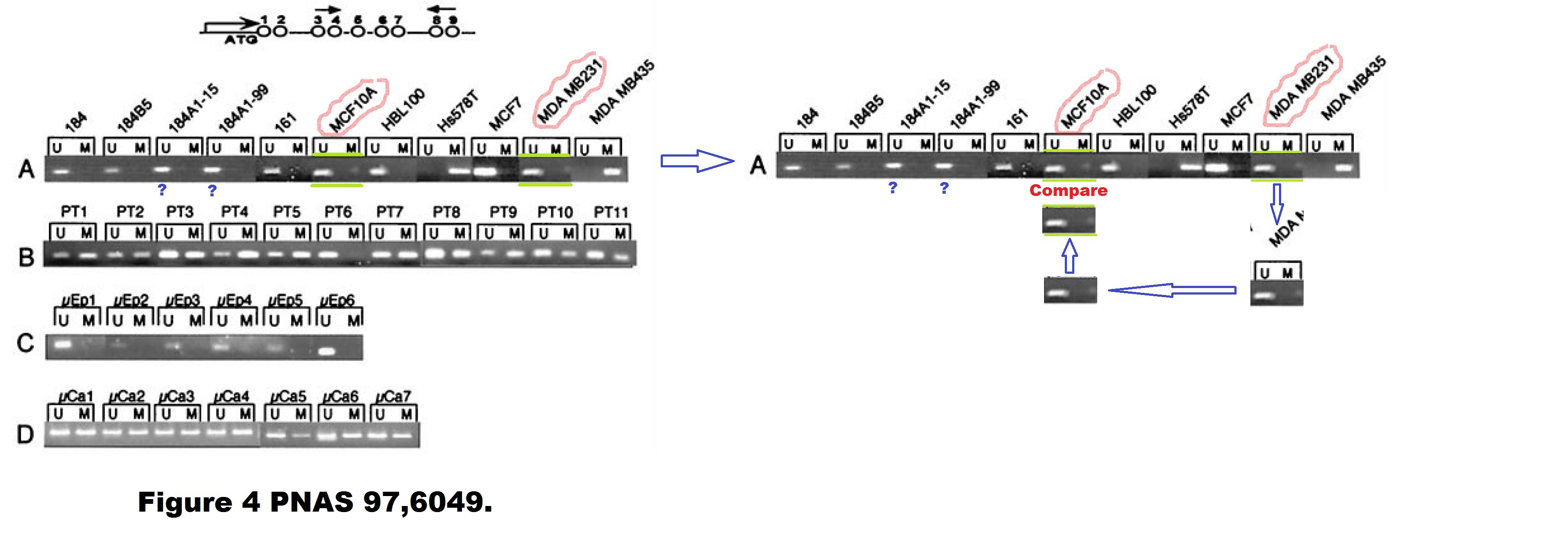
Professor Hermeking had no comments on that one. All four allegedly continuous gels of this figure are most obviously spliced from various pieces, allow me to visualise it:
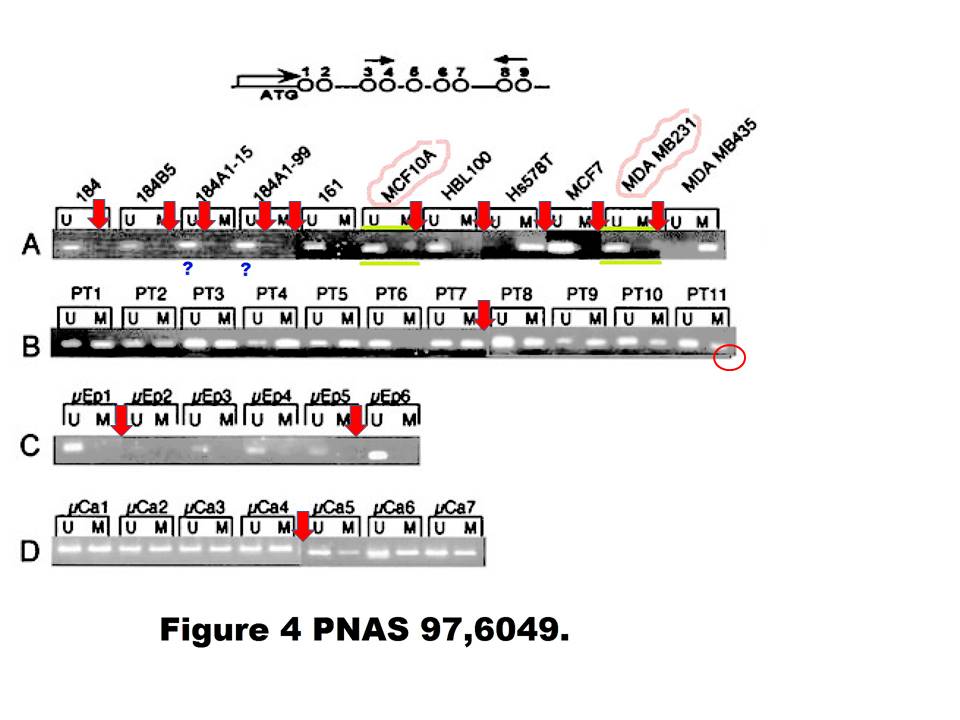
Under these circumstances, the case for the two suspected duplications is much stronger than the case against. There is even a corner (red circle) where one additional gel image was digitally overlaid on top of the original gel image. This is not splicing as such anymore, but something much more problematic. I am puzzled why Professor Hermeking is reluctant to find and name the culprit here.
Here are two old Hermeking papers where it is not exactly clear what the scientific rationale for the (undeclared) gel lane splicing was.
Regarding the 1995 JBC paper, it was the PhD student, as Hermking states:
“Work of a PhD student (Franz Kohlhuber) in the laboratory of my supervisor (Dirk Eick). The position where the membrane/gel/autoradiography was cut is clearly visible in my opinion. The data in itself is not “manipulated” by this, irrelevant results were removed from the figure.“
I presume the splicing in the PNAS 2000 paper was done by some PhD student in Vogelstein’s lab?
Most recently, this paper was flagged by the pseudonymous Clare Francis:
Helge Siemens , Jens Neumann , Rene Jackstadt , Ulrich Mansmann , David Horst , Thomas Kirchner , Heiko Hermeking Detection of miR-34a promoter methylation in combination with elevated expression of c-Met and β-catenin predicts distant metastasis of colon cancer Clinical cancer research (2013) doi: 10.1158/1078-0432.ccr-12-1703
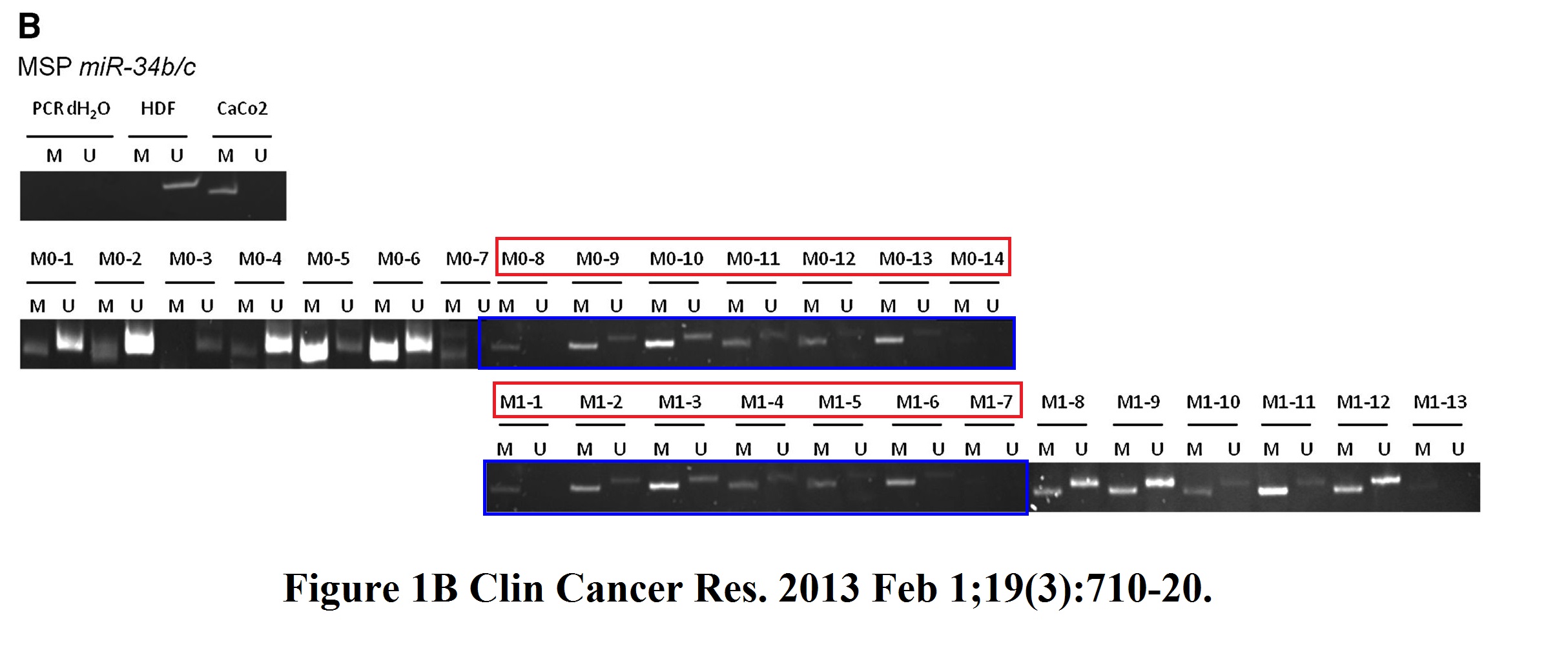
I asked Hermeking which PhD student was it this time, he replied:
“I will discuss this with the doctoral student at the time, Helge Siemens.
However, Fig. 1B (miR-34b/c methylation) is irrelevant for the statement of the publication. Like in result text, we have focused all further analyzes in this publication on miR-34a methylation, since miR-34b/c is associated in almost all tumors, whether with (M1) or without (M0).distant metastasis, is methylated. We had found this in other studies as well.
However, this should not be an excuse for this figure.
We will of course try to work on a corrigendum for this.”
I also think all these figures, and most likely the papers they are part of, are all irrelevant. Anyway, I don’t know about LMU Munich, but Johns Hopkins University has probably enough of these PubPeer antics.
Contact Form
I thank all my donors for supporting my journalism. You can be one of them!
Make a one-time donation:
I thank all my donors for supporting my journalism. You can be one of them!
Make a monthly donation:
Choose an amount
Or enter a custom amount
Your contribution is appreciated.
Your contribution is appreciated.
DonateDonate monthly



Fresh problematic data for Heiko Hermeking:
PubPeer – miR-34a and IRE1A/XBP-1(S) Form a Double-Negative Feedback L…
LikeLiked by 1 person
Great job, I just sent it to LMU to cover up and whitewash
LikeLike
Fresh problematic data for Heiko Hermeking:
PubPeer – Characterization of a p53/miR-34a/CSF1R/STAT3 Feedback Loop…
LikeLiked by 1 person
Heiko Hermeking on board. 10 authors. Perhaps it is normal.
PubPeer – Tissue-specific opposing functions of the inflammasome adapt…
LikeLiked by 1 person