“PubPeer may have had a role at some point, but as far as I can tell, is now a platform for resentful, anonymous, petty, failed scientists to harass those who actually make discoveries“
Professor David M Sabatini, mTORman
David Sabatini is MIT professor and a titan of molecular biology, an absolute superstar or actually, a superhero of science. The Whitehead Institute principal investigator calls himself mTORman, because he is credited with the discovery of the mTOR kinase, which regulates many cellular processes and is therefore an invaluable target for the pharma industry.

mTORman’s chief superhero power is apparently to be able to certify all conclusions of his top-rank papers as unaffected, despite image duplications therein, and without ever looking into raw data. Instead, Sabatini sends his first authors to replace the offending figures and to warn everyone not to question their papers. And those who insist he checks the data consistency anyway are described as “steaming turds”. Those like Strobilanthes Asper who recently raised concerns about data integrity in Sabatini’s papers, get off lightly: they are merely “failed scientists”. Apparently, these days this is the dictionary definition of
Scientist, successful: a person employed at elite research institution, able to regularly place papers in journals with highest impact factor despite data irregularities and unverifiable research results. Is generally above scrutiny from journals and universities; instils the fear of God in critics.
Some time ago, Sabatini’s science was mildly criticised on PubPeer, and back then no concerns of data integrity were raised. The great man was not amused. The Harvard superstar, who gets angry easily and throws expletives at his critics, protested the anonymity of a PubPeer commenter. After all, a man is entitled to have revenge, and not knowing your critic’s identity is not helpful here.
I think Strobilanthes Asper did a great job flagging Sabatini’s papers on PubPeer and would like to open with a particularly disastrous one. The MIT superstar is penultimate author there, meaning his lab contributed the second-biggest share to that paper. The last author is Dos Sorbassov, former mentee of Sabatini, who then moved to MD Anderson in Texas (a notorious place), and who is now back in his home country Kazakhstan as professor at the Nazarbayev University (named after the country’s undead post-Soviet long-term dictator).
D Boulbes, CH Chen, T Shaikenov, NK Agarwal, TR Peterson, TA Addona, H Keshishian, SA Carr, MA Magnuson, DM Sabatini , DD. Sarbassov Rictor phosphorylation on the Thr-1135 site does not require mammalian target of rapamycin complex 2 Molecular cancer research : MCR (2010) doi: 10.1158/1541-7786.mcr-09-0409
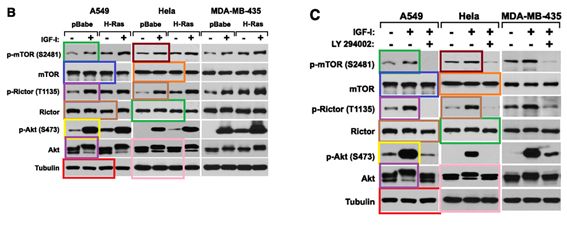
Horrible, right? An orgy of Photoshop fraud, a retraction is too mild for this awful paper. So far, mTORman saw no reason to jump to action.
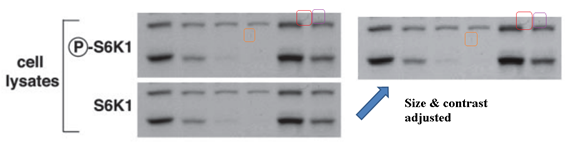
This makes you suspicious of another Sarbassov & Sabatini JBC 2005 cooperation, where a gel for total protein looks exactly like the phosphorylated one (they are supposed to look somewhat similar in band shapes, but never identical). Or, is this really an innocent mistake of oversight, spotted by another PubPeer commenter in an old Science paper from Sabatini lab:
D D Sarbassov, DA Guertin, SM Ali, DM Sabatini Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex Science (2005) doi: 10.1126/science.1106148
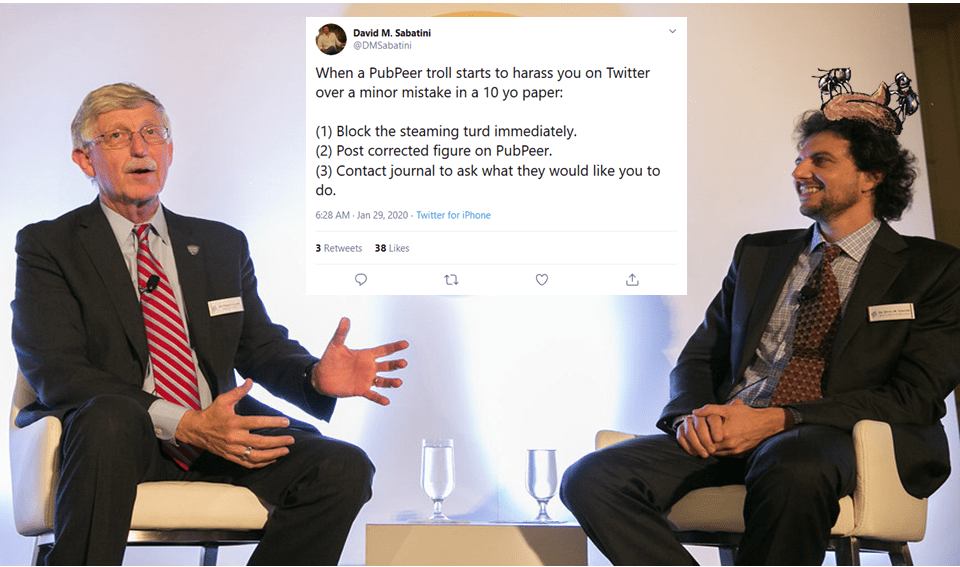
Again, S6K1 blots are accidentally duplicated. As if there was some kind of bug with the S6K1 data? Sarbassov’s papers are truly worth studying, more evidence is listed in the comments below this article. To be fair, Sabatini reacted immediately after I pointed him to his Boulbes et al 2010 paper and the next one, in Nature: he blocked me on Twitter and described me as a “steaming turd”. Which I presume also precludes any possible inquiries at Whitehead and MIT?
N Kanarek , HR. Keys , JR. Cantor , CA. Lewis , SH Chan , T Kunchok , M Abu-Remaileh , E Freinkman , LD. Schweitzer , DM. Sabatini Histidine catabolism is a major determinant of methotrexate sensitivity Nature (2018) doi: 10.1038/s41586-018-0316-7
Three image duplications inside same Figure 11, a and b. A rather new paper, why did nobody notice that? Or was Nature afraid to tell Sabatini, fearing he will publish with Cell again next?
The first author Naama Kanarek, now group leader in Harvard, sees it all very relaxed:
“we will also ask Nature if they wish to correct it on the online version of the paper. We emphasize that this mistake does not change the conclusion of the figure or any of the statements made in the paper.“
When Harvard and MIT folks keep telling you such things, it does sound a bit like a threat. The paper is not even 2 years old, and they already warn you not to ask for a correction, apparently exactly because this is in Nature. whom they also expect to do as told. Can someone tell our scientific elites it is not for them and their editor buddies to decide when their shoddy attitude to data presentation affects the conclusions, but for the scientific community? Especially since they refuse to examine the raw data or to test the reproducibility of their results, as Sabatini made clear on Twitter?
How does one recover old data stored somewhere in Sabatini lab via email discussions with first author who moved on to open a lab of their own? No wonder the hastily procured replacements do not seem to exactly fit. Here is the next figure for Dr Kanarek of Harvard to fix, in that same Nature 2018 paper:
Turns out, the quantifications presented in Figure 2 are based on copy-pasted numbers, as evidenced by the raw data authors had to supply according to new Nature guidelines. Does this affect any of the conclusions, Dr Kanarek?


Kanarek had replacement data ready and explained:
“In my attempt to make the source data files clear I transposed each of the data sets and named the sets by cell line, treatment and metabolite. It was a mistake because it caused multiple errors.“
Nada Y. Kalaany , David M. Sabatini Tumours with PI3K activation are resistant to dietary restriction Nature (2009) doi: 10.1038/nature07782

The coauthor Nada Kalaany, now associate professor at Dana Farber / Harvard Cancer Institute replied on PubPeer and announced a possible correction:
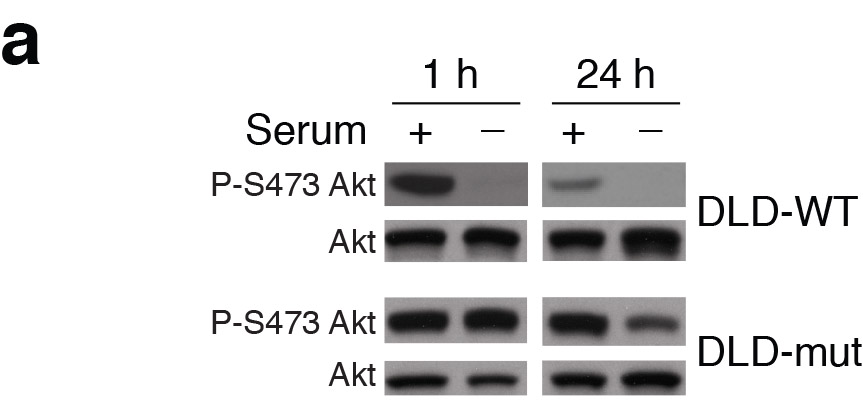
“This error does not impact the conclusions of the paper. Nevertheless, we will contact the Nature Editorial Office to let them know about this mistake and its correction.“
Where the replacement gel exactly came from is not clear, the new phospho-AKT band “smiles” upwards, while the total AKT band “smiles” downwards, a rare thing to happen were it the same gel re-probed. The new replacement blot also has a different colouring, as another PubPeer commenter noticed. All of which lets one wonder where exactly Dr Kalaany got it from.
In the next instance, a confocal microscopy image was reused in a different case first in a bioRxiv preprint, and then in a PNAS paper. The experimental settings were very different. Sabatini was penultimate, Jared Rutter of University of Utah last author.
CK Kikani , X Wu , S Fogarty , SA Woo Kang , N Dephoure , SP Gygi, DM. Sabatini , J Rutter Activation of PASK by mTORC1 is required for the onset of the terminal differentiation program Proceedings of the National Academy of Sciences (2019) doi: 10.1073/pnas.1804013116
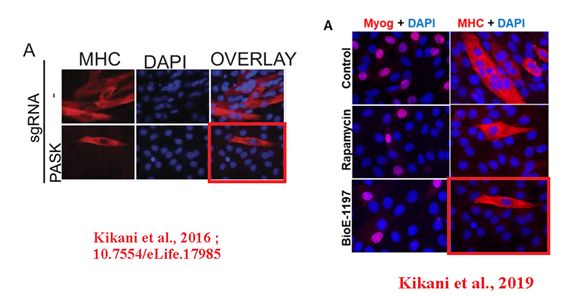
First author Chitnan Kikani is now assistant professor at University of Kentucky, and he announced to fix both the preprint and the PNAS paper:
“we wish to point out that our erroneous insertion of a panel does not aid or affect data interpretation in any way. […] We are now pursuing the submission of an erratum, wherein we will explain the error and replace the image with the appropriate one.“
The next duplication is also puzzling.
J Wedel , S Bruneau , K Liu , SW Kong , PT. Sage , DM. Sabatini , M Laplante, DM. Briscoe DEPTOR modulates activation responses in CD4 + T cells and enhances immunoregulation following transplantation American journal of transplantation (2019) doi: 10.1111/ajt.14995

The first author Johannes Wedel, originally from Germany, is faculty member in the Harvard lab of David Briscoe, who is last author of that paper. Wedel explained on PubPeer, “on behalf of all authors”:
“this minor error and its correction (see below) does not affect the summary of n=8 mice/group illustrated in the same Figure (Fig.1F). This likely resulted from a copy and paste issue during the preparation of the figure and went unnoticed during the submission, review and publication of the manuscript. Also, importantly this minor error and its correction does not change the interpretation of data or conclusions drawn from the extensive additional analyses (including full transcriptomic data, in vitro and in vivo functional analyses) illustrated in the other figures. Nevertheless, we have notified the Editorial Office of the American Journal of Transplantation about this error and its correction.“
Maybe not so fast, Herr Doktor Wedel. Surely everyone can accidentally reuse a FACS dataset, but: Since the quantifications are different (11.9 vs 18.0), the most benevolent explanation would be that someone was routinely analysing flow cytometry samples of the same experiment with different gate settings.
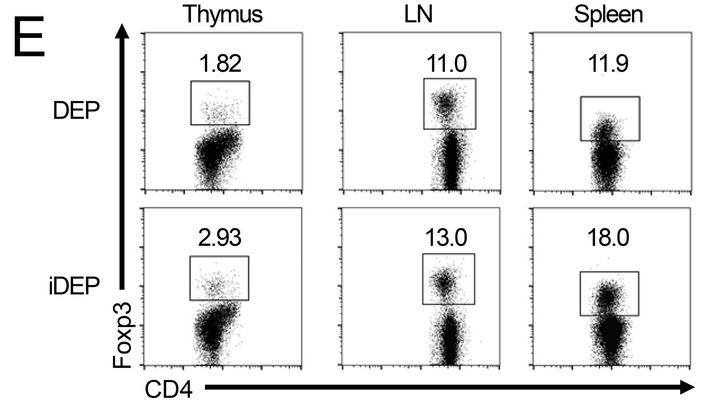
A very bad and inappropriate practice (even if other German experts see it differently). Miraculously, Wedel’s replacement figure had the same quantification number 11.9, now based on Ersatz flow cytometry plot (right).
It is not reassuring that neither Briscoe nor Sabatini nor other elite labs have a problem with such approach to flow cytometry. On the other hand, it does produce the “right” results for the right journals. The Journal of Biological Chemistry (JBC) is however the wrong journal to put bent data in.
Q Liu , S Kirubakaran , W Hur , M Niepel , K Westover, CC. Thoreen, J Wang , J Ni , MP. Patricelli , K Vogel , S Riddle , DL. Waller , R Traynor , T Sanda , Z Zhao , SA. Kang , J Zhao , AT Look, PK. Sorger , DM. Sabatini , NS. Gray Kinome-wide selectivity profiling of ATP-competitive mammalian target of rapamycin (mTOR) inhibitors and characterization of their binding kinetics Journal of Biological Chemistry (2012) doi: 10.1074/jbc.m111.304485
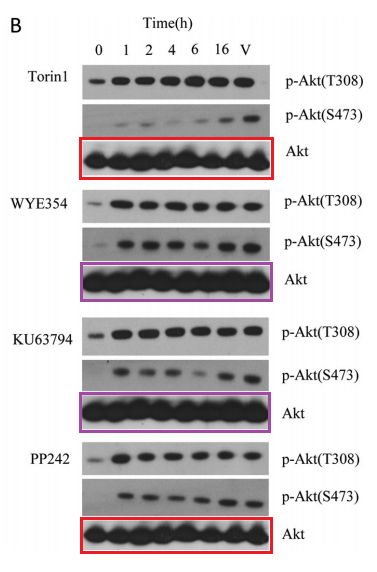
That figure was originally flagged for the re-used Akt western blots, which function as loading control here. A mistake of oversight maybe, until someone else commented:
“Akt panels appear to show 8 lanes as a control for a 7 lane blot“
Oops. This suggest that the authors possibly did not have the correct Akt blots at hand in the first place, and used some unrelated library loading controls, copy-pasted two times each, without even bothering if they at least show the same number of samples.
But how do we know the blots were equally loaded then? Simple: because the last authors are Sabatini and Nathaniel Gray of Dana Farber Cancer Institute in Harvard. QED. This is also why the first author of another Sabatini-Gray co-production Carson Thoreen (now associate professor at not just somewhere, but at Yale) replaced a western blot loading control in Thoreen et al JBC 2009 while warning:
“We would like to emphasize that these changes have no impact on any of our original conclusions.“
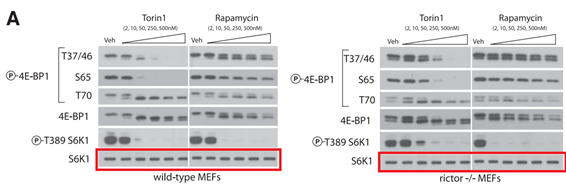
Another commenter pointed out that “the background color of this new blot is slight different than all the other blots (is more blueish, while the others are gray)“, which lets one wonder again where the resourceful first author got it from. If it was the original, correct scan from 2009, it would have been the same colour as the rest of the figure, no?
Yale professor Thoreen would probably also say none of that affect the conclusions of this paper of his:
Y Sancak , CC. Thoreen , TR. Peterson , RA. Lindquist , SA. Kang , E Spooner , S A. Carr, M. Sabatini PRAS40 is an insulin-regulated inhibitor of the mTORC1 protein kinase Molecular Cell (2007) doi: 10.1016/j.molcel.2007.03.003
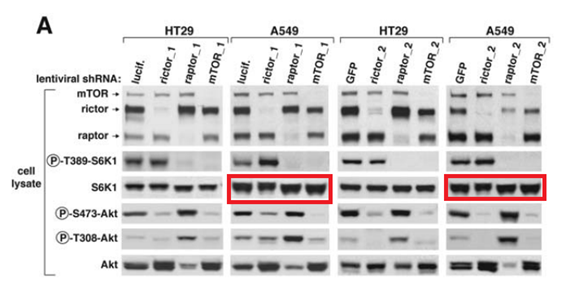
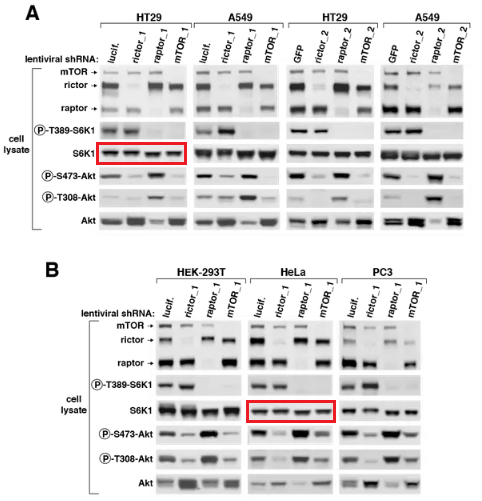
First author Yasemin Sancak is now assistant professor at University of Washington. What is it with that wretched ribosomal protein S6 kinase S6K which always needed some tinkering with?
This other Sabatini collaboration is another chapter in 50 shades of Gray, very painful and excruciating to watch:
Q Liu , C Xu , S Kirubakaran , X Zhang , W Hur , Y Liu , NP Kwiatkowski , J Wang , KD Westover , P Gao , D Ercan , M Niepel , CC Thoreen , SA Kang , M P Patricelli , Y Wang , T Tupper , A Altabef , H Kawamura , KD Held , DM Chou, SJ Elledge, PA Janne, KK Wong, DM Sabatini, NS Gray Characterization of Torin2, an ATP-competitive inhibitor of mTOR, ATM, and ATR Cancer Research (2013) doi: 10.1158/0008-5472.can-12-1702
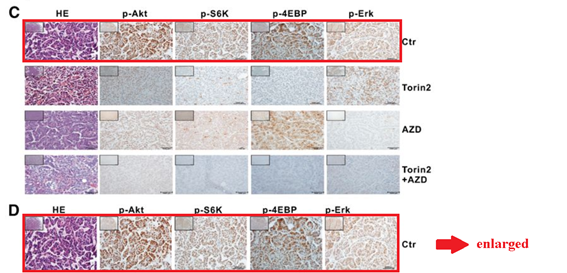
Somehow the histological sections for animals sacrificed after 2 days of treatment (Figure 5C) are identical to those sacrificed after 4 weeks (Figure 5D), only stretched. A mistake? Sure it is, just like these duplications are, once again that S6K protein misbehaved:
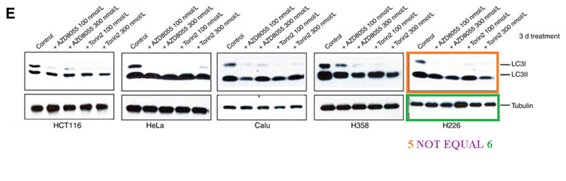
The two papers are merely 8 years old, but so far the authors remained silent. There is little incentive for Gray to bother with PubPeer criticisms in the first place, because the director of his Ludwig Center at Dana Farber is none other by Bob Weinberg, who is a god in his own right and as such above such things.
Speaking of gods. Before forming an opinion about this Cell paper from the lab of David Root at Broad Institute, you should ask yourself: are you worthy? It features the cancer research god Bill Hahn and the Broad Institute director Eric Lander, next to Sabatini. On your knees, now.
J Moffat, DA. Grueneberg, X Yang, SY Kim, AM Kloepfer, G Hinkle, B Piqani, TM Eisenhaure, B Luo, K. Grenier, AE Carpenter, SY Foo, SA. Stewart, BR. Stockwell, N Hacohen, WC Hahn, ES Lander, DM Sabatini, DE Root A lentiviral RNAi library for human and mouse genes applied to an arrayed viral high-content screen Cell (2006) doi: 10.1016/j.cell.2006.01.040
The first author Jason Moffat, now professor at University of Toronto, replied:
“We are checking into this.”
Moffat did not say whether the conclusions are affected or not, but in any case: only a steaming turd of a failed scientist would doubt his Cell paper now.
The next paper has a minor duplication of a FACS plot, but the Korean first author Joon-Ho Sheen is presumably too busy to reply, being a senior scientist at LG.
JH Sheen , R Zoncu , D Kim , DM. Sabatini Defective Regulation of Autophagy upon Leucine Deprivation Reveals a Targetable Liability of Human Melanoma Cells In Vitro and In Vivo Cancer Cell (2011) doi: 10.1016/j.ccr.2011.03.012
Sabatini, in whose lab the original data remained, cannot be bothered. In 2021 that paper will be 10 years old and it will be inappropriate to ask him to check for any eventual raw data anyway.
Also here nobody bothered to reply. The first author Liron Bar-Peled is now assistant professor and principal investigator at Dana Farber/Harvard Cancer Center.
L Bar-Peled, LD. Schweitzer, R Zoncu, David M. Sabatini Ragulator is a GEF for the rag GTPases that signal amino acid levels to mTORC1 Cell (2012) doi: 10.1016/j.cell.2012.07.032
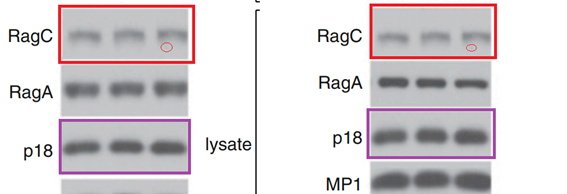
This time it does not look like a mistake of oversight. The Figures 1A and 1B contain 12 western blots each, labelled as clearly different assays with different protein constructs. And yet the RagC and p18 panels were reused, and stretched, which made them look dissimilar.
There are other issues with Sabatini’s publication record: a collaborative paper with a Chinese group contains an unashamedly fake western blot, or a shady paper with the notorious Italian cheater duo Carmine Settembre and Andrea Ballabio (Settembre et al EMBO J 2012).
More recently, concerns were raised that the authors misinformed peer reviewers and readers about the specificity of the NEK10 antibody central to the paper’s main findings: the paper Chuvukula et al Nature Medicine 2020 is brand new. Similar concerns were noted for a slightly younger Nature paper from Sabatini lab, Wolfson et al Nature 2017. But the mighty mTORman already made clear on Twitter that he does not intend his papers for the scientific community, only for the exclusive readership of editors at elite journals. Whether or not the presented scientific results are any reliable, does not in any way affect the perfectly factual and reproducible conclusions that those were published in Nature and Cell.
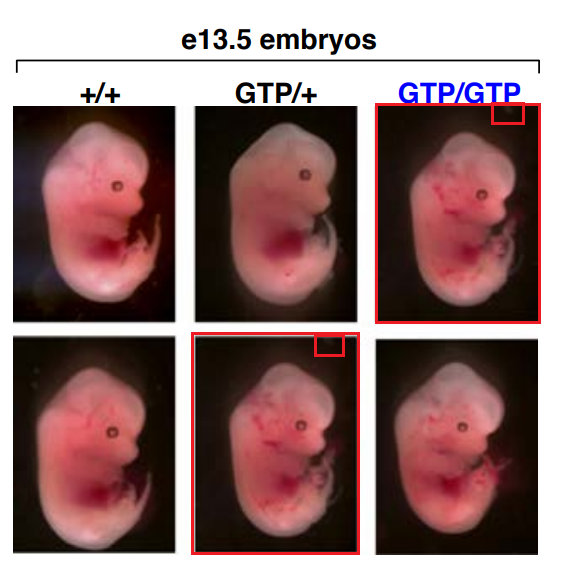
There is also a duplicated picture of a mouse embryo in Sabatini’s own paper in Nature, Efeyan et al 2013, co-authored by his dad, David Sabatini Sr. There, the first author Alejo Efeyan, now group leader at CNIO in Madrid, informs everyone:
“It is important to note that neither this very minor mistake nor its correction affect the validity of the data, its interpretation, conclusions drawn, or any other aspect of the published paper.“
You steaming turds have been warned.

This article was updated on 30 and 31 January 2020.
Update 21.08.2021
Sabatini was sacked after an investigation found that he “violated the Institute’s policies on sexual harassment among other Whitehead policies unrelated to research misconduct”.
An email by Whitehead Institute director Ruth Lehmann was shared with the employees:
I am writing to let you know that David Sabatini, a member of Whitehead Institute, is no longer associated with either the Whitehead Institute or the Howard Hughes Medical Institute, effective immediately.
Dr. Sabatini’s departure comes on the heels of his receipt of a report laying out the findings of an independent investigation into the culture and working environment of his lab. This investigation was precipitated by a Diversity, Equity and Inclusion survey commissioned and conducted last winter which collected data and comments on the culture across the Institute. The results of this survey identified issues of particular concern in the Sabatini Lab and that led to the appointment of Hinkley Allen & Snyder LLP to investigate the Sabatini Lab. In sum, the investigation found that Dr. Sabatini violated the Institute’s policies on sexual harassment among other Whitehead policies unrelated to research misconduct.
Dr. Sabatini’s departure has significant implications for the 39 members of his lab, four of whom are HHMI employees; the remainder are Whitehead employees. Whitehead human resources personnel will be conducting one-on-one meetings with all 39 – next week – to help effectuate a plan to ensure their smooth transition to another lab setting so that they may continue their work in pursuit of their career goals.
I am and will always be steadfast in my commitment to providing an inclusive, supportive environment for the training and research of our community.Ruth Lehmann
Maybe the misconduct investigation set off other complaints. Pier Paolo Pandolfi was also sacked in Harvard for sexual harassment unrelated to research misconduct.
That’s what we steaming turds achieved.
For more mTOR fraud, read here:
Donate!
If you are interested to support my work, you can leave here a small tip of $5. Or several of small tips, just increase the amount as you like (2x=€10; 5x=€25). Your generous patronage of my journalism will be most appreciated!
€5.00






Latest developments in Whitehead Institute/Sabatini affair: https://newsopener.com/science/whitehead-institute-seeks-pause-on-prominent-biologists-defamation-lawsuit/ .
LikeLike
Pingback: The Sex Privileges of mTORman David Sabatini – For Better Science
Massive correction on Sabatini’s paper published today by Nature…
https://www.nature.com/articles/s41586-021-03487-2
LikeLike
“In Fig. 1f of this Article, one of the three biological replicates of the control drug doxorubicin was erroneously duplicated from another experiment. […]
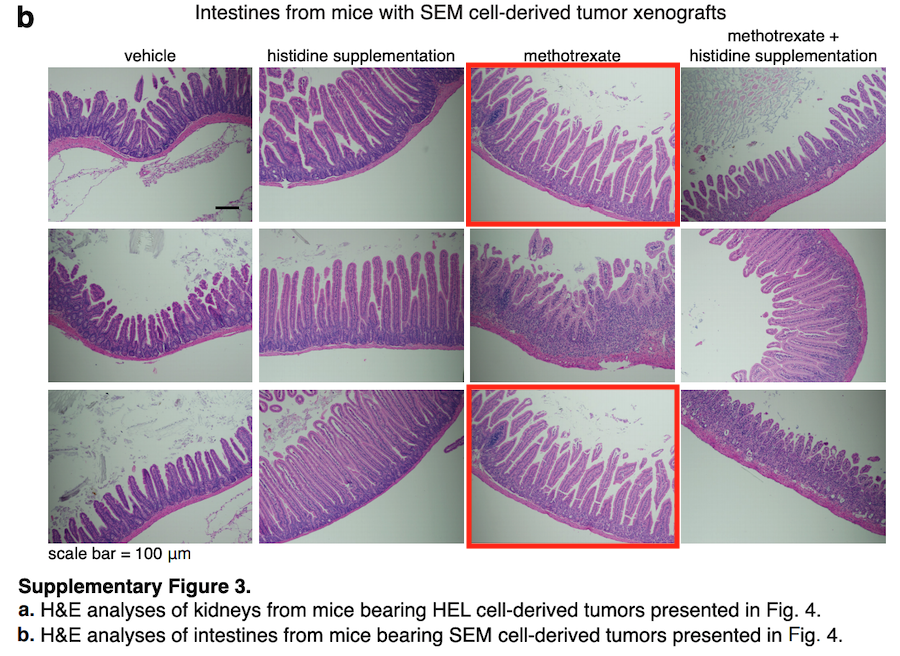
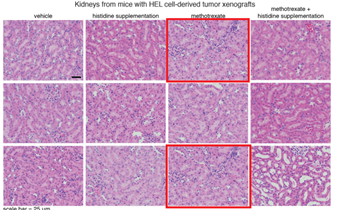
In Extended Data Fig. 11 of this Article, two of the histological sections in the ‘methotrexate’ group (top and bottom images in Extended Data Fig. 11a) and two sections in the ‘vehicle’ group (middle and bottom images in Extended Data Fig. 11b) were duplicated, and the sections in the ‘histidine supplementation’ (bottom image in Fig. 11b) and ‘methotrexate’ (top image in Extended Data Fig. 11b) groups were also duplicated. […]
In Supplementary Fig. 3 of this Article, two of the histological sections in the ‘methotrexate’ group were duplicated. […]
There were errors in the Source Data of Fig. 2 that have been corrected and have been highlighted in red in Supplementary Information to this Amendment; the data in the original Fig. 2 were correct. In the Methods section, the reference for the sgRNA library that was used for the screen (Wang et al. (2014), ref. 7) was incorrect, and the updated library was instead from Wang et al. (2015)”
BUT: “These errors do not affect the conclusions of the paper”
From experience I can tell you why this paper was corrected and not retracted. The first author is now a Harvard PI, her career must be protected:
“David Sabatini is no longer affiliated with the Whitehead Institute or the Howard Hughes Medical Institute. At the request of the Whitehead Institute and to ensure execution of the duties of corresponding author, the corresponding author on this Author Correction is now Naama Kanarek (naama.kanarek@childrens.harvard.edu), replacing David Sabatini.”
LikeLike
“These errors do not affect the conclusions of the paper”.
I’ve asked here before how it can be that people–scientists!–say that with a straight face. But it has just occurred to me that, perhaps, the standard for ‘truth’ in scientific research is what the lawyers call “the preponderance of the evidence”? I mean, I guess that’s always been the case on a large scale: lots of papers are published, eventually consensus is reached (not necessarily permanently!), prior publications contradicting that consensus are (ideally) studied to see where they went wrong, and so on. Onwards and upwards! But maybe now it’s on the level of a single paper, or the output of a single lab? No positive statement in a paper need be thought of as indispensable to the conclusion!
My slowness in forming this hypothesis is undoubtedly due to my being a mathematician.
LikeLike
Here is finally the correction to one of the mentioned articles. https://www.nature.com/articles/s41586-021-03487-2
LikeLike
https://www.science.org/content/article/prominent-biologist-david-sabatini-out-mit-after-breaching-sexual-relationship-policy
“David Sabatini, the high-profile biologist who was forced out of the Whitehead Institute in summer 2021 after a probe found he violated its sexual harassment policies, has resigned his tenured professorship at the Massachusetts Institute of Technology (MIT). His move came after three senior MIT officials recommended revoking his tenure.”
Dateline is April 1 but I don’t think it’s a joke…
LikeLike
Lesson Nr 1: never sue your employer when they try to sack you quietly and honourably.
LikeLike
That’s what we steaming turds achieved.
If it was “unrelated to research misconduct”, then it wasn’t you who achieved it.
LikeLike
Yeah, I am sure NIH was about to give Sabatini a multimillion grant. Pity for them NYU didn’t employ him.
LikeLike
https://www.uochb.cz/en/news/561/a-new-scientific-group-is-being-established-at-iocb-headed-by-american-scientist-dr-david-sabatini
LikeLike
It seems, Sabatini has a new engagement:
https://www.uochb.cz/en/news/561/a-new-scientific-group-is-being-established-at-iocb-headed-by-american-scientist-dr-david-sabatini
LikeLike