This post by Viola Sheltonii and yours truly serves to congratulate Mansoor Amiji, professor at Northeastern University in Boston, USA, on his election as Fellow to the National Academy of Inventors. He is the perfect scientist to Make America Great Again.
The announcement came on 12 December 2025:
“In his more than three decades at Northeastern, Amiji has played a leading role in the development of vaccine delivery systems and pharmaceutical technologies designed to treat cancers, neurodegenerative conditions, gastrointestinal diseases and more. […]
One recent project Amiji and his team worked on that is ready for clinical trials is an oil-droplet-based drug delivery system used to attack cancerous cells in the body. He’s also just recently developed treatments for brain cancer and neurological conditions such as Parkinson’s and Alzheimer’s.
And he has plenty more, explained Harris, who in his nomination letter highlighted that Amiji has collaborated with more than 25 pharmaceutical companies on clinical trials throughout his decades-long career and has co-founded several biotechnology companies himself.
“Amiji is a rare gem; his skills and character make him a modern-day polymath cut in the mold of the great scientists who defined the natural sciences at the turn of the 1990s,” Harris wrote. “
The university announcement doesn’t say who this Harris was, but who cares. Amiji is the hero here, a genius born in Tanzania, who made a stellar career in USA since he arrived in 1983. Amiji’s PubPeer record definitely proves his inventor’s skills, 31 badly forged papers, but the professor disagrees.
Amiji’s case certainly reminds of another great titan of nanotechnology and nanotheranostics at Northeastern University – Thomas Webster. His fraud was covered up and defended, while the university celebrated every new brainfart by Webster (picotechnology, anyone?) as a stroke of genius, just as they do with Amiji. Eventually Northeastern sacked Webster, who then became a full-time papermiller.
Thomas Webster to save the world with COVID-19 nanoparticles
The world is in the grip of COVID-19 pandemic. Thousands dead, infection rates explode, nations in lockdown. Perfect timing for troll scientists to offer their bullshit cures. Like Thomas Webster of Northeastern University.
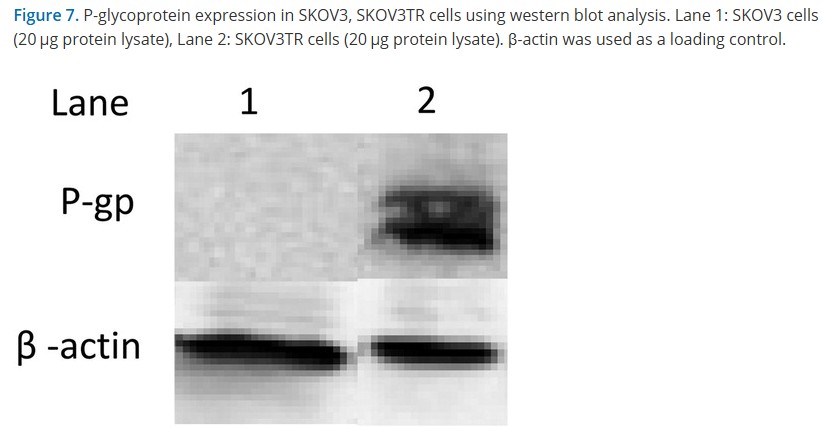
Let’s start with a study where Amiji cured cancer with nanoparticles made from gelatin. Amiji’s former PhD student Goldi Kaul is since 2021 Vice President at the pharma giant Boehringer Ingelheim:
Goldie Kaul , Mansoor Amiji Tumor-targeted gene delivery using poly(ethylene glycol)-modified gelatin nanoparticles: in vitro and in vivo studies Pharmaceutical Research (2005) doi: 10.1007/s11095-005-4590-3
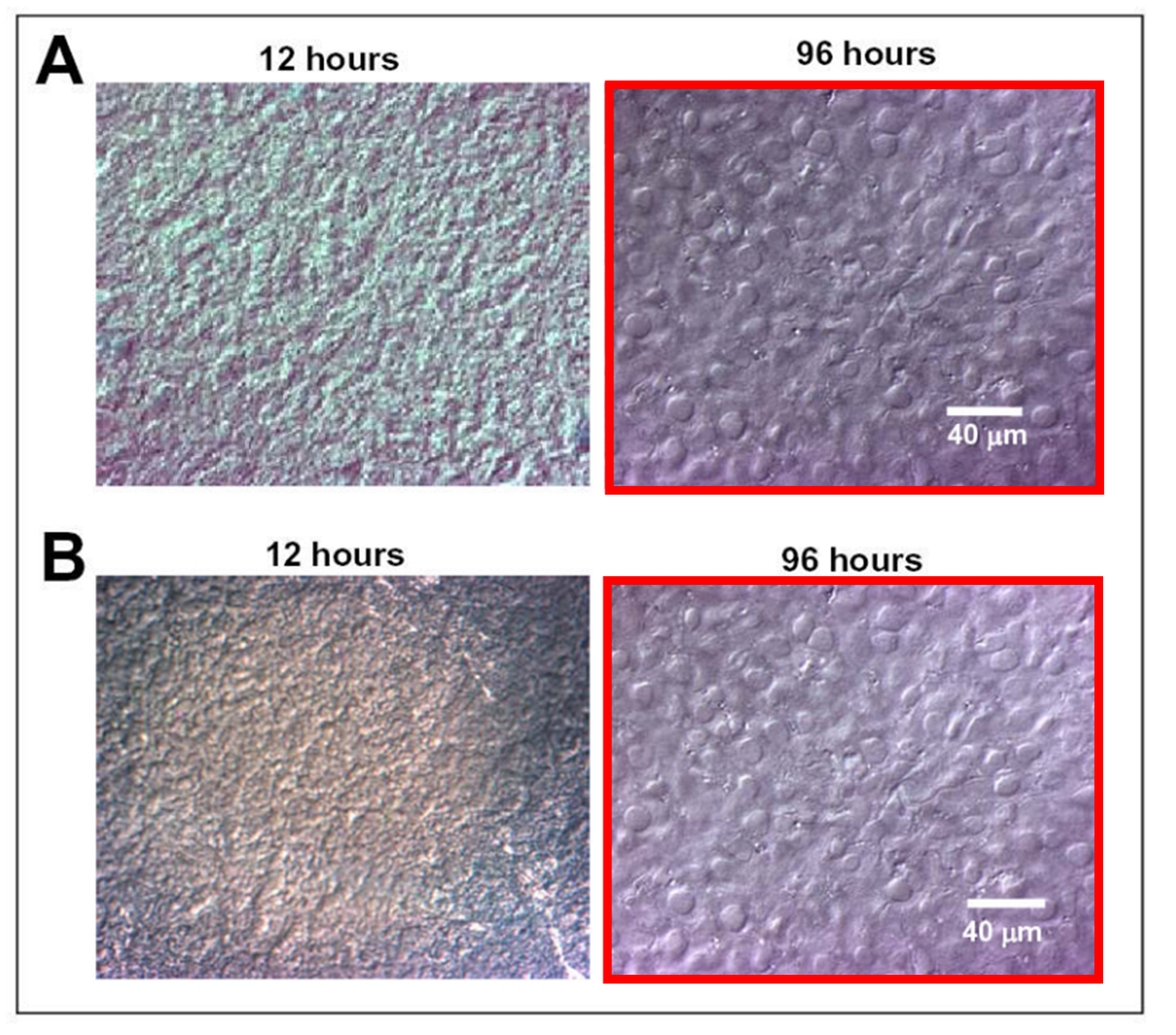
Gelatin is good, but how else is one to cure cancer than with curcumin?
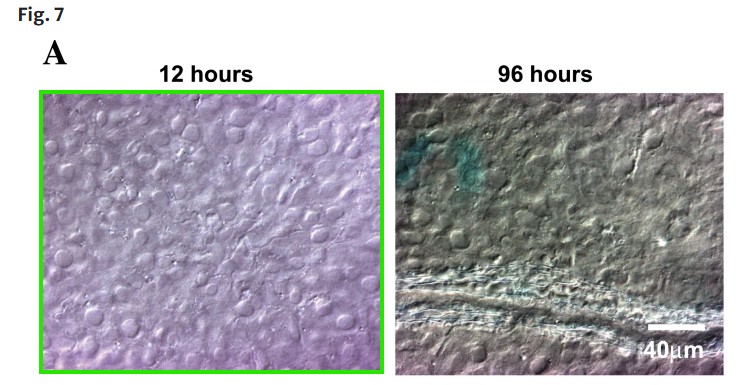
Srinivas Ganta , Harikrishna Devalapally , Mansoor Amiji Curcumin enhances oral bioavailability and anti-tumor therapeutic efficacy of paclitaxel upon administration in nanoemulsion formulation Journal of Pharmaceutical Sciences (2010) doi: 10.1002/jps.22157
Also, no trash science without chitosan:
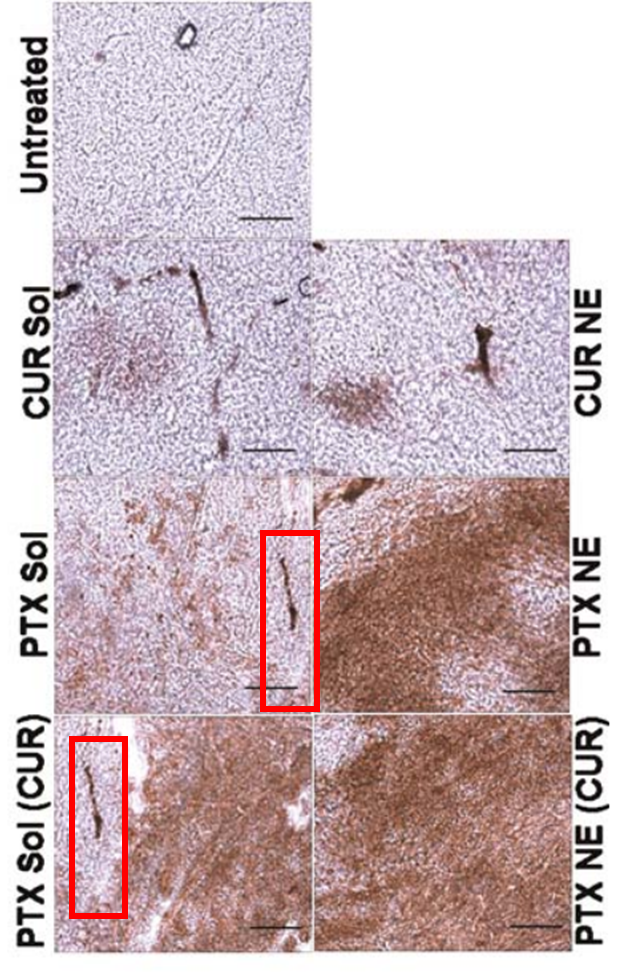
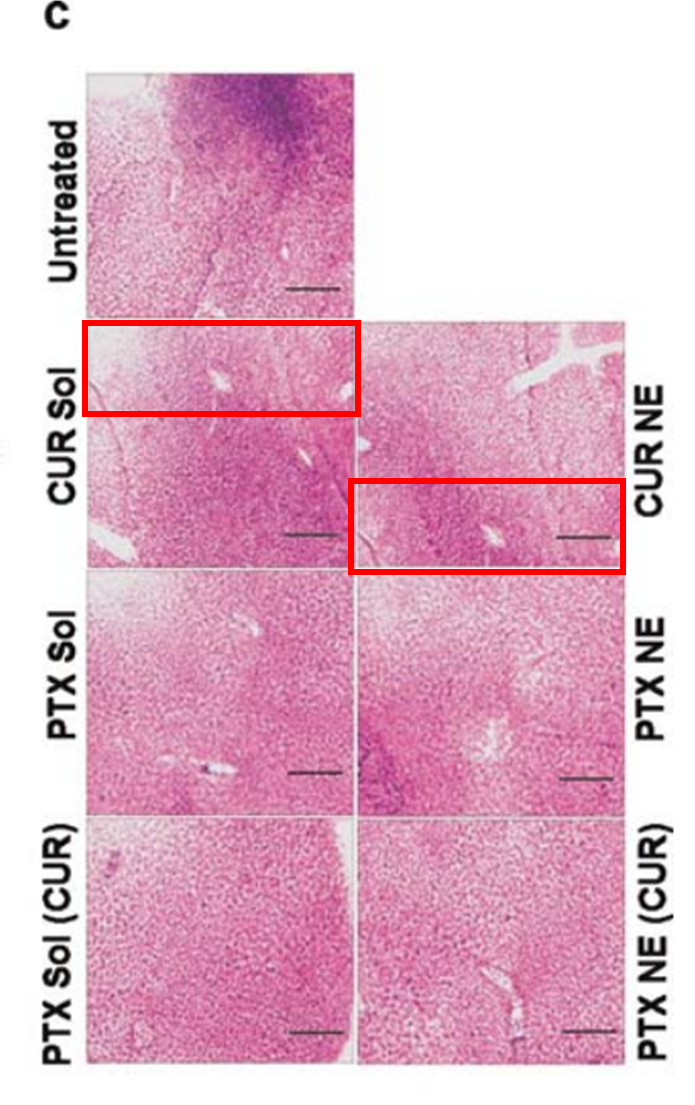
Ana Vanessa Nascimento , Amit Singh , Hassan Bousbaa , Domingos Ferreira , Bruno Sarmento , Mansoor M. Amiji Overcoming cisplatin resistance in non-small cell lung cancer with Mad2 silencing siRNA delivered systemically using EGFR-targeted chitosan nanoparticles Acta biomaterialia (2017) doi:10.1016/j.actbio.2016.09.045

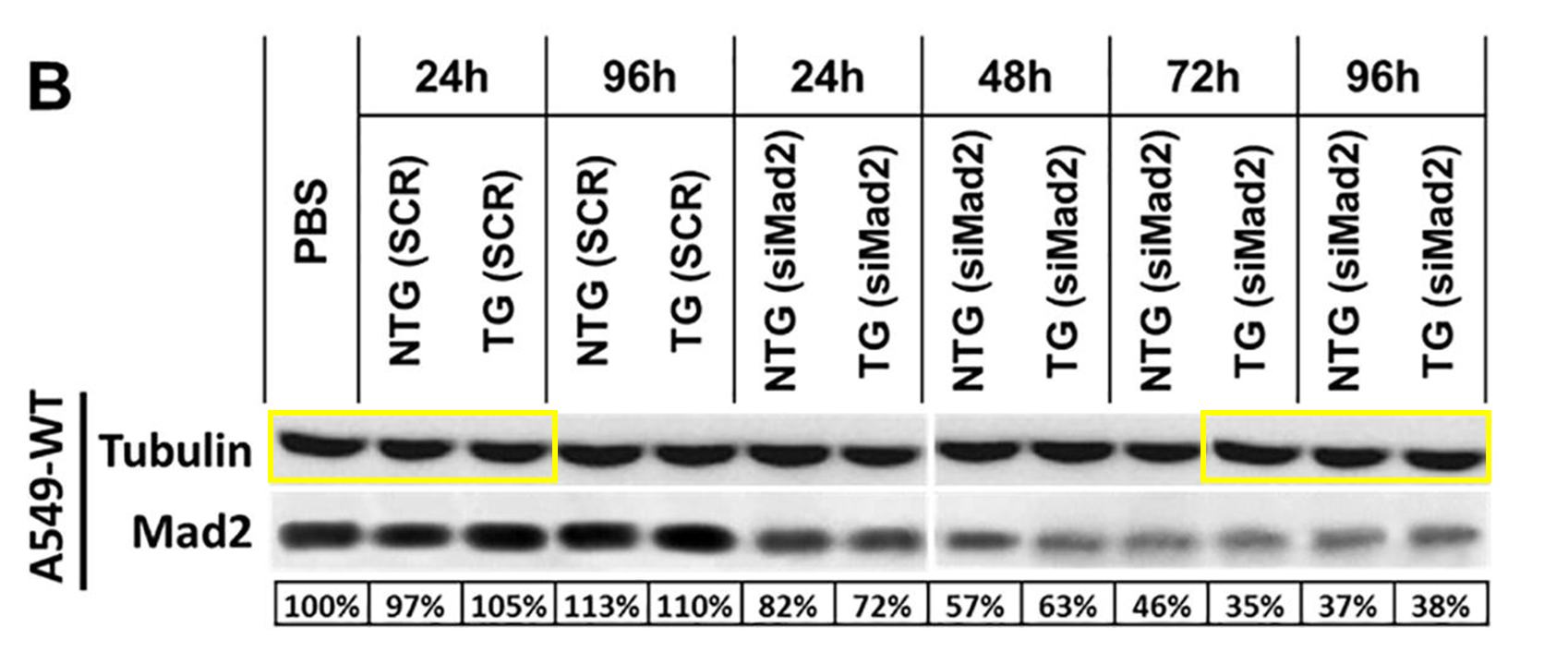
Northeastern University once claimed that Amiji’s work was “Kryptonite for cancer cells“. Here is more bad cancer research by our Superman:
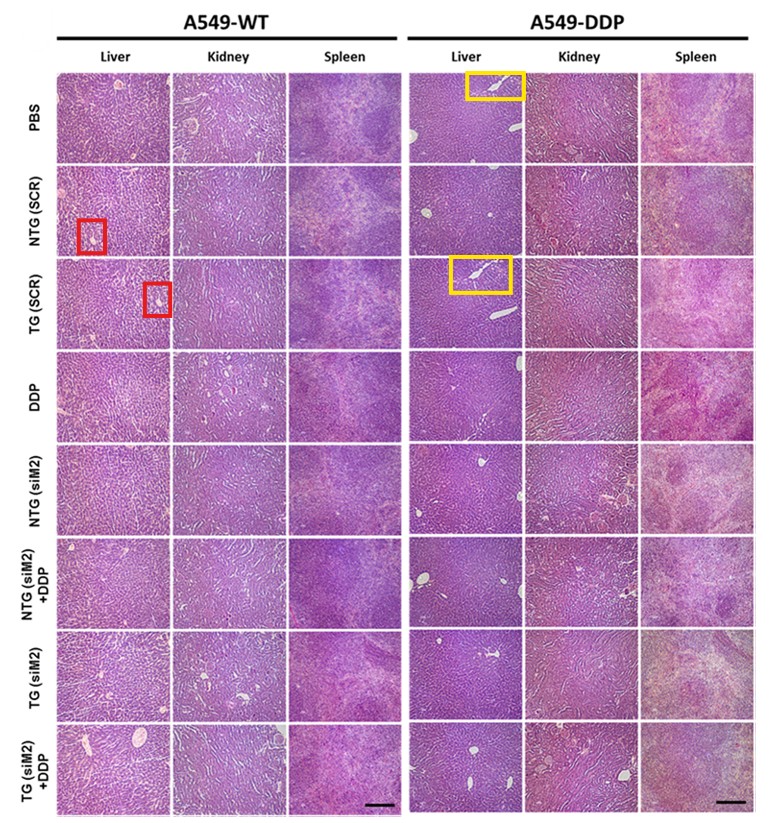
Meghna Talekar, Qijun Ouyang , Michael S. Goldberg, Mansoor M. Amiji Cosilencing of PKM-2 and MDR-1 Sensitizes Multidrug-Resistant Ovarian Cancer Cells to Paclitaxel in a Murine Model of Ovarian Cancer Molecular Cancer Therapeutics (2015) doi: 10.1158/1535-7163.mct-15-0100
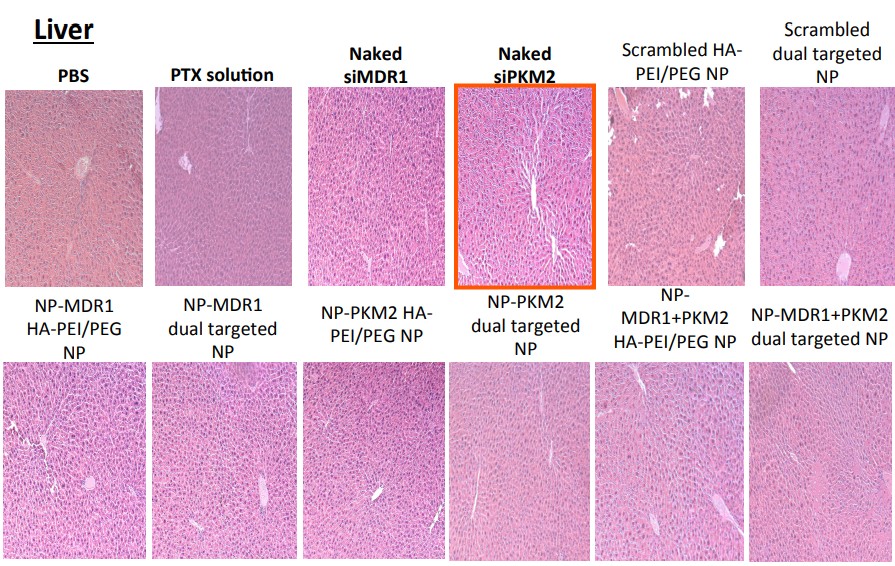
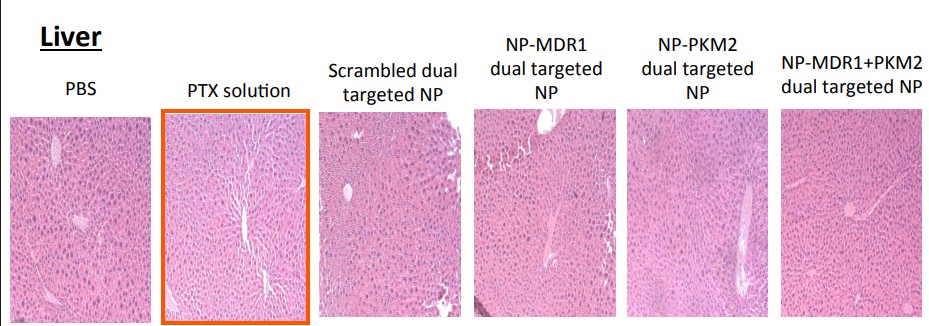
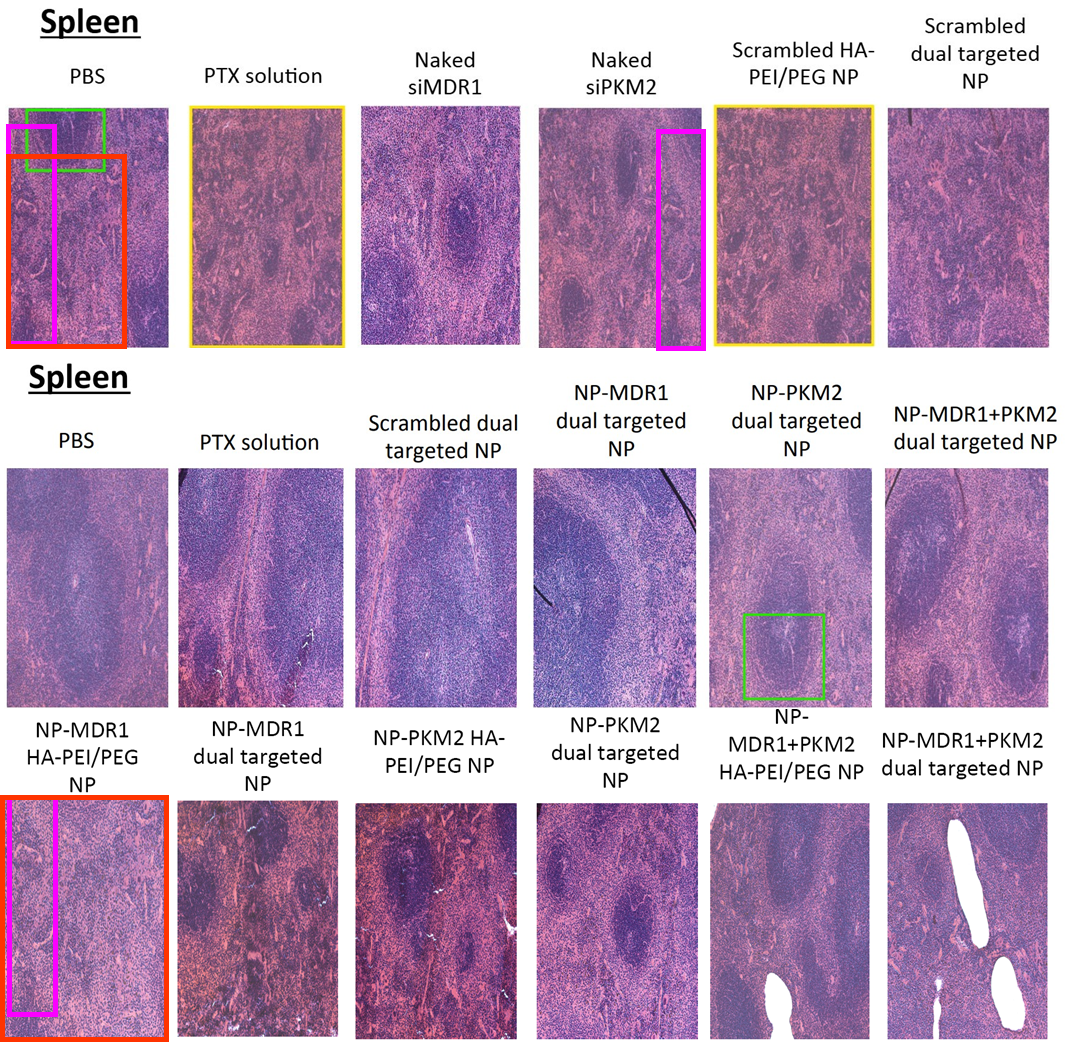
In August 2025, Amiji replied on PubPeer and warned the sleuths: “None of these images are duplicated“. They only looked similar because the organs of “control and test animals and look similar“. The man is a professor, who are we to argue.
Amiji was also criticised for animal abuse. See Sousa et al 2019, or this study:
Amit Singh , Jing Xu , George Mattheolabakis, Mansoor Amiji EGFR-targeted gelatin nanoparticles for systemic administration of gemcitabine in an orthotopic pancreatic cancer model Nanomedicine : nanotechnology, biology, and medicine (2016) doi: 10.1016/j.nano.2015.11.010
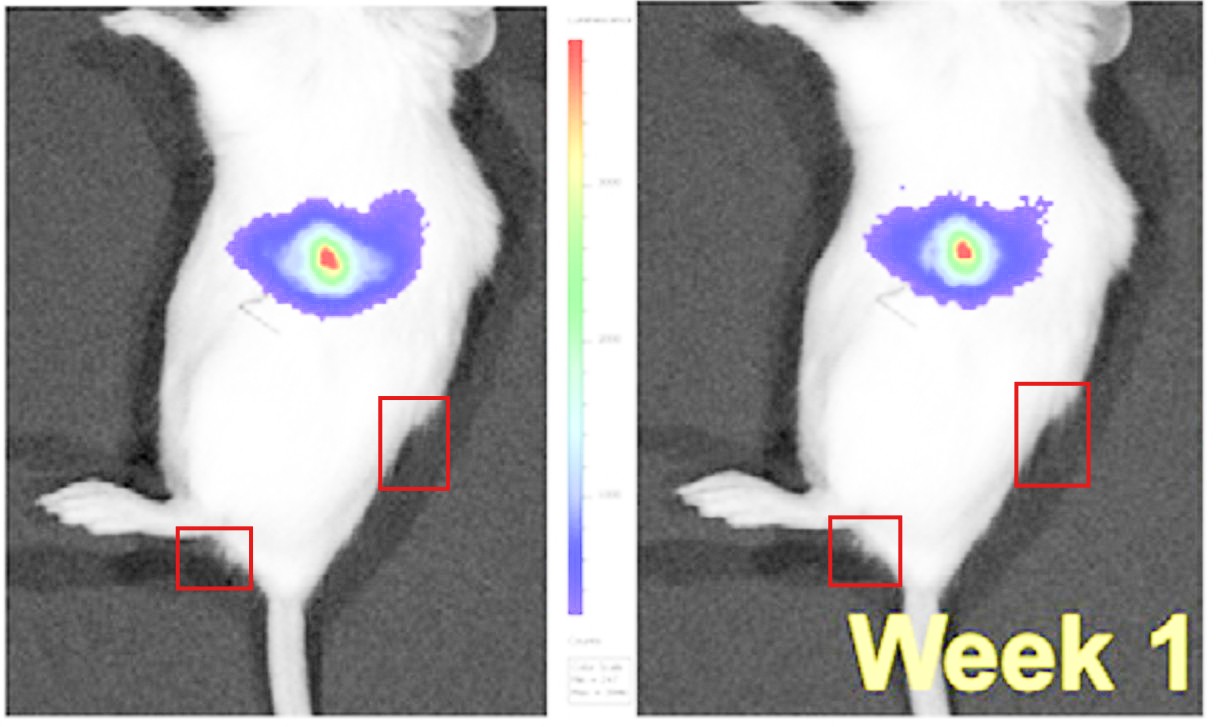
Viola sheltonii :”Figure 6A appears to show the same mouse with different bioluminescence.”
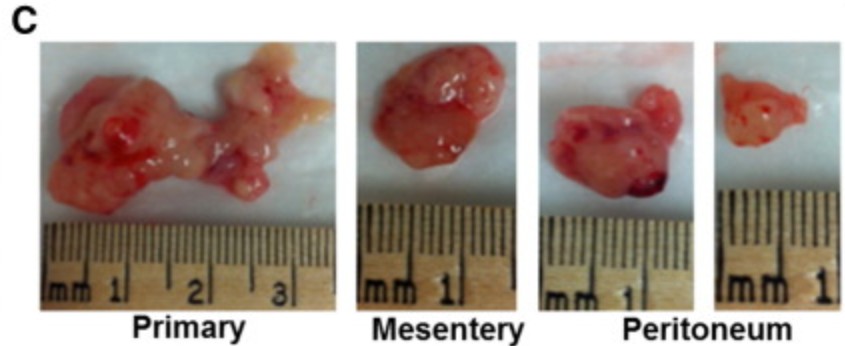
The sleuth Viola sheltonii asked Amiji to provide an ethics approval for his unethical mouse experiments with tumour xenografts. In July 2025, he replied on PubPeer with a claim that those experiments were approved, and anyway:
“the tissue in the image is not all tumor but also healthy tissue, which leads one to believe that the size of the tumor is 3.0 cm when, factually it is not.“
Actually, no, his own figure legend says: “C) Image of primary tumors excised
from the site of injection and secondary tumor nodules excised from mesentery and peritoneum“, also the main text refers to “multiples metastatic tumor nodules along with the primary tumor at the site of injection (Figure 6C).”
The Lies of Mice
“The closer I looked, the worse it was. Not just because it’s sad to think too much about the poor lives of a laboratory mouse, but also because the researchers who used these mice didn’t have the decency to be truthful about their poor lives. ” – Viola Sheltonii
As for the duplicated mouse images, Amiji insisted that “it is not the same mouse” and the pixel-identical nature of the two images actually proves the “reproducibility of the tumor growth for 1 week and 4 week time points“. This was the first response from Amiji on PubPeer, despite 14 earlier comments regarding duplicated and/or fabricated images.
More cloned mice here, plus forged gels. This approach to science qualified Amiji’s mentee Shardool Jain to become “Due Diligence Lead” in pharma industry:
Shardool Jain , Thanh-Huyen Tran , Mansoor Amiji Macrophage repolarization with targeted alginate nanoparticles containing IL-10 plasmid DNA for the treatment of experimental arthritis Biomaterials (2015) doi: 10.1016/j.biomaterials.2015.05.028
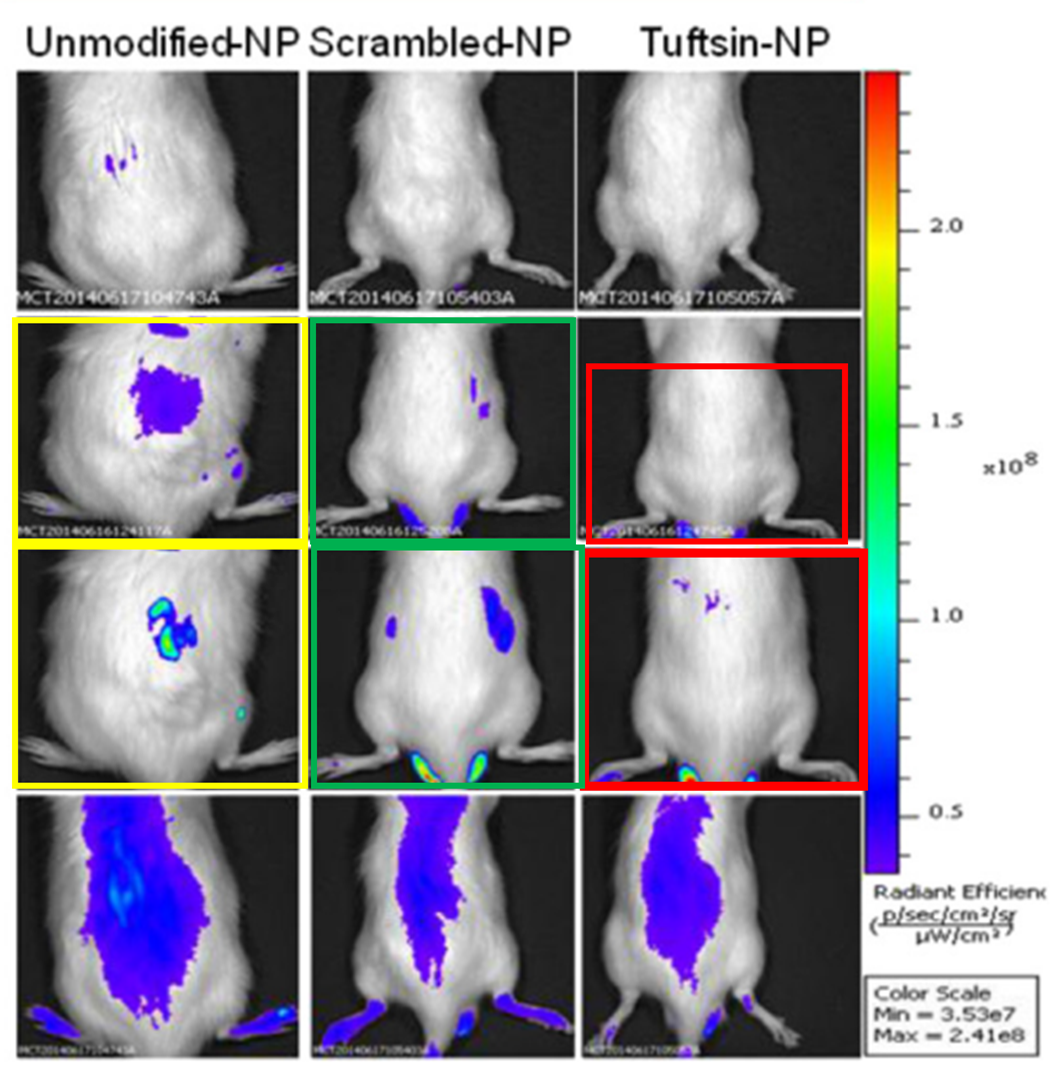
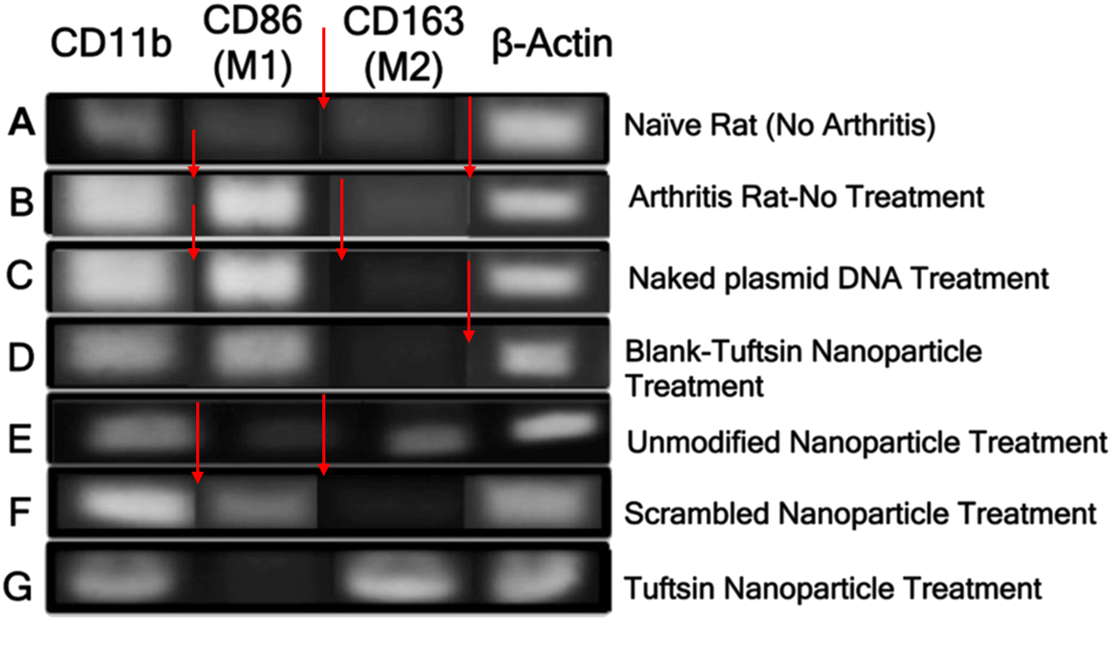
Here, Amiji warned again that “none of the images are duplicated” and the PCR bands are meant to look identical since he wanted to see no difference between “arthritis bearing rats and those treated with naked IL-10 plasmid DNA“.
The great inventor had nothing to say regarding the splice lines throughout Figure 4, because Amiji only has something to say when someone brings up duplicated images – only 6 out of~35 papers on PubPeer have warranted a response from him. Usually in the form of, paraphrased: “these are not duplicated because I used Microsoft Paint to say they are from two different timepoints therefore they are different.”
Similar denials for duplicated mice in Nascimento et al 2016, and now look what else Amiji managed to deny. His coauthor Aliasgar Shahiwala is now professor in the Emirates and author of many books about nanotechnology:
Aliasgar Shahiwala , Mansoor M. Amiji Enhanced mucosal and systemic immune response with squalane oil-containing multiple emulsions upon intranasal and oral administration in mice Journal of Drug Targeting (2008) doi: 10.1080/10611860801900082
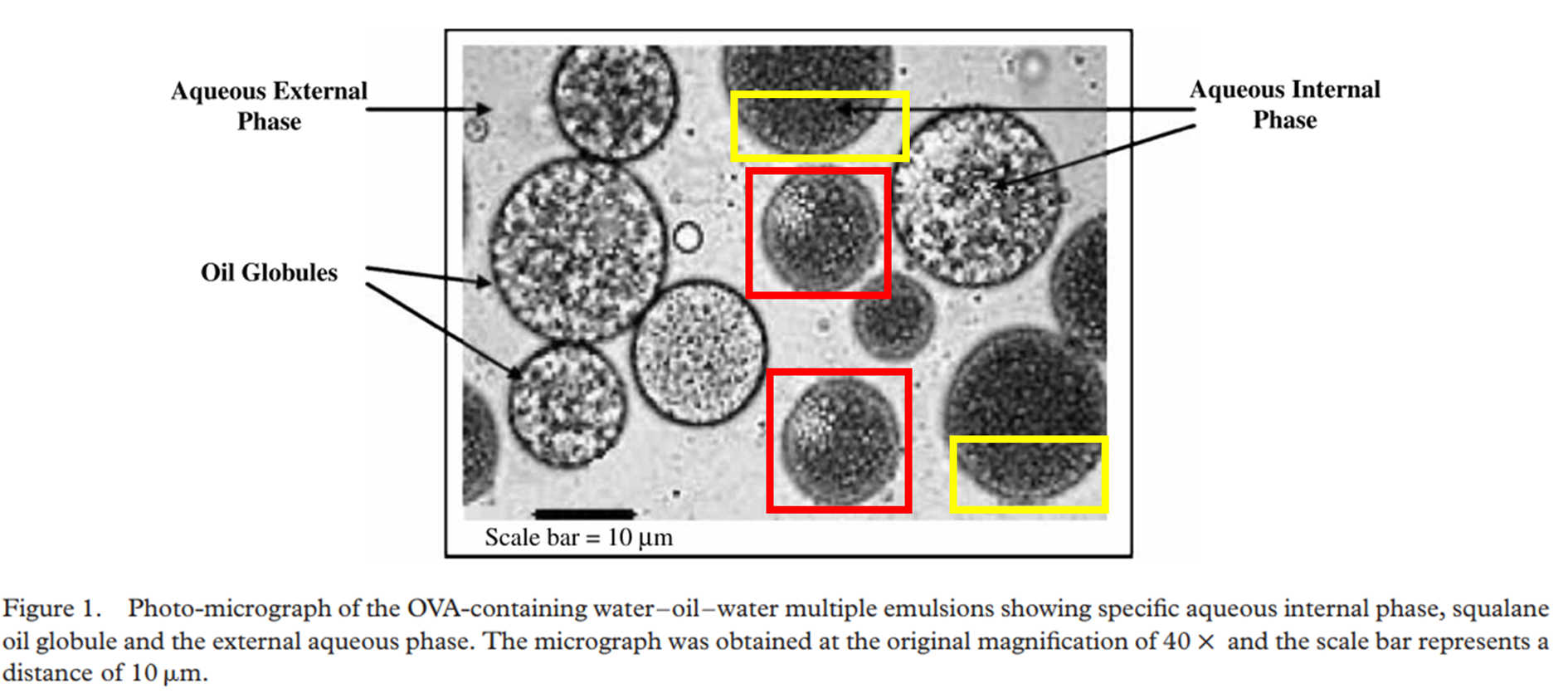
In July 2025, Amiji announced on PubPeer to be “fully committed to upholding the integrity of the scientific record“, then proceeded to warn that the figure “did not serve as the basis for any of the conclusions“, and anyway, the particles only look similar because the “standard room light” in 2007 allowed only for “low resolution“.
Yes, this is an Academician from an elite university in USA talking. With over 30 fake papers on PubPeer.
In Amiji’s world there are never any identical images unless journals make him admit to it. This was corrected:
Gulzar Ahmad , Rana El Sadda , Galina Botchkina , Iwao Ojima , James Egan , Mansoor Amiji Nanoemulsion formulation of a novel taxoid DHA-SBT-1214 inhibits prostate cancer stem cell-induced tumor growth Cancer Letters (2017) doi: 10.1016/j.canlet.2017.08.004
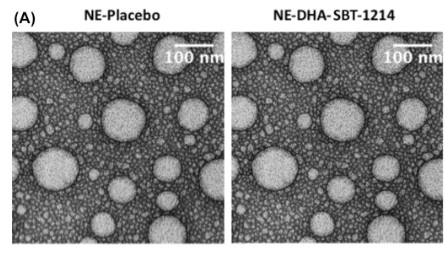
Viola sheltonii: “Figure 1 shows the same image for NE-Placebo and NE-DHA-SBT-1214”
The Correction appeared just 2 months later, on 24 September 2025:
“After the publication of this article, it was brought to the authors’ attention that in Fig. 1A (Characterization of DHA-SBT-1214 nanoemulsion formulation.), The transmission electron micrograph (TEM) of the placebo (NE-Placebo) and DHA-SBT-1214 nanoemulsion formulation (NE-DHA-SBT-1214) images were identical. After comparing with the original TEM data, the authors found those two images belong to DHA-SBT-1214 nanoemulsion. The corrected image for the transmission electron micrograph (TEM) of the placebo (NE-Placebo) and DHA-SBT-1214 nanoemulsion formulation (NE-DHA-SBT-1214) are shown as below. The analysis, results and conclusions of the manuscript remain valid. The authors apologize for this error.”
Fake-O-Meat by Ali Khademhosseini
Ali Khademhosseini is the greatest American researcher in regenerative medicine. His mentees are all professors themselves now. In his own Californian institute, he grows not only all possible organs, but even hamburgers!
Here Amiji also had to admit duplication, his coauthor is the MIT superstar Robert Langer (former mentor of the disastrous Ali Khademhosseini):
Anupama Potineni , David M Lynn , Robert Langer , Mansoor M Amiji Poly(ethylene oxide)-modified poly(beta-amino ester) nanoparticles as a pH-sensitive biodegradable system for paclitaxel delivery Journal of Controlled Release (2003) doi: 10.1016/s0168-3659(02)00374-7
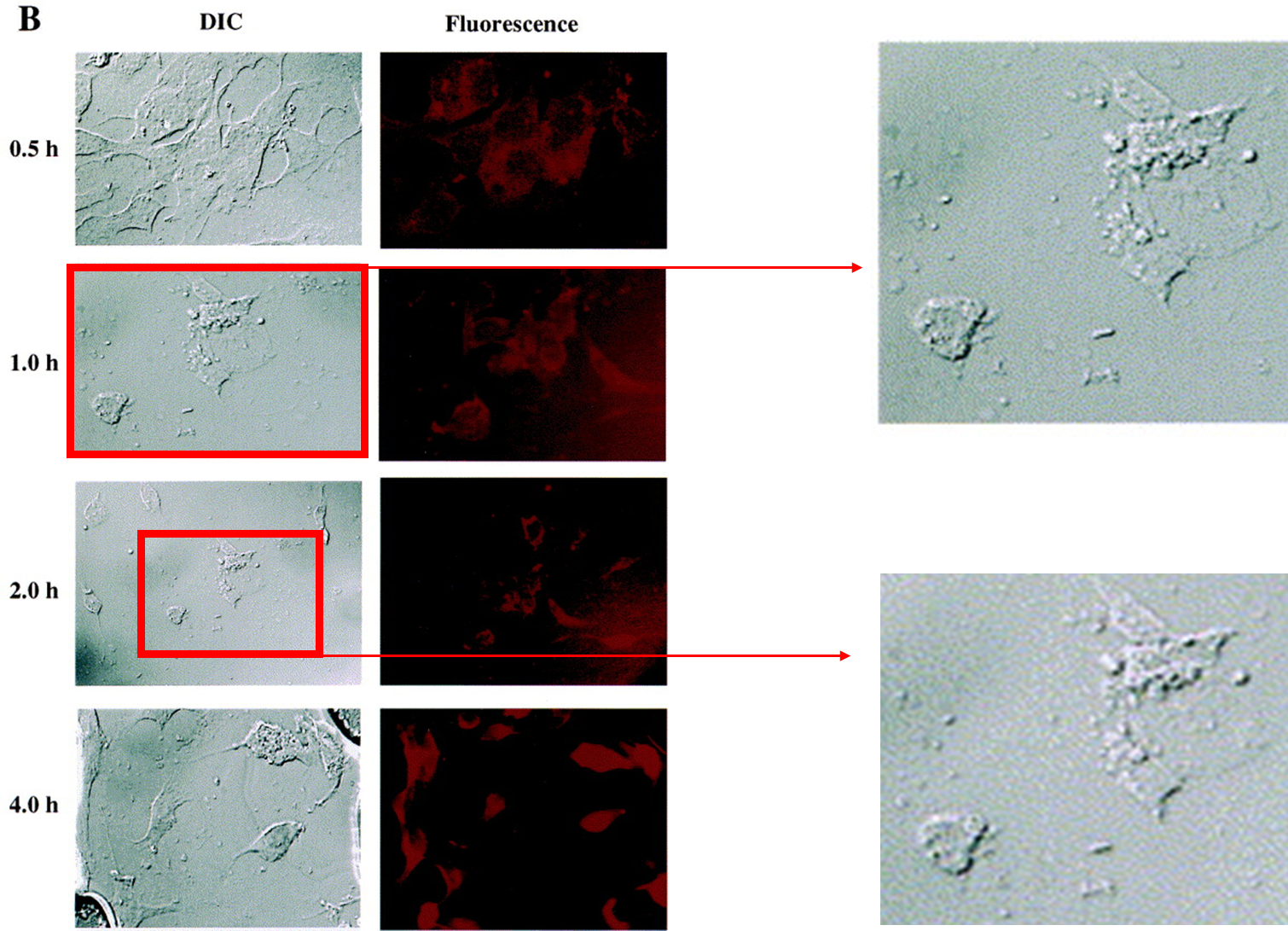
The Corrigendum from November 2025 was somewhat unusually written:
“Description of Error: In Fig. 7B shows differential interference contrast (DIC) and fluorescence confocal images of rhodamine-123-containing poly(ethylene oxide)-modified poly-1 nanoparticle uptake and intracellular distribution in BT-20 human breast cancer cells as a function of time. The DIC images on the left for 1 h and 2 h time points were duplicated in error. The fluorescence images on the right are correct for each of the time points. Unfortunately, this study is over two decades old, and we do not have the correct DIC images available today to replace.
Impact Assessment: This error affects only the visual presentation in Fig. 7B for the 1 h and 2 h time points and does not impact on the scientific premise, data analysis, conclusions, or interpretation presented in the manuscript. The experimental results and discussion remain valid.
The authors would like to apologise for any inconvenience caused.”
See, if the authors and editors haven’t kindly provided this “Impact Assessment” to guide your thinking, you would have thought all the wrong thoughts.
We also got a kind of impact assessment here:
Nicusor Iftimia, Arun K. Iyer , Daniel X. Hammer , Niyom Lue , Mircea Mujat , Martha Pitman , R. Daniel Ferguson , Mansoor Amiji Fluorescence-guided optical coherence tomography imaging for colon cancer screening: a preliminary mouse study Biomedical Optics Express (2012) doi: 10.1364/boe.3.000178
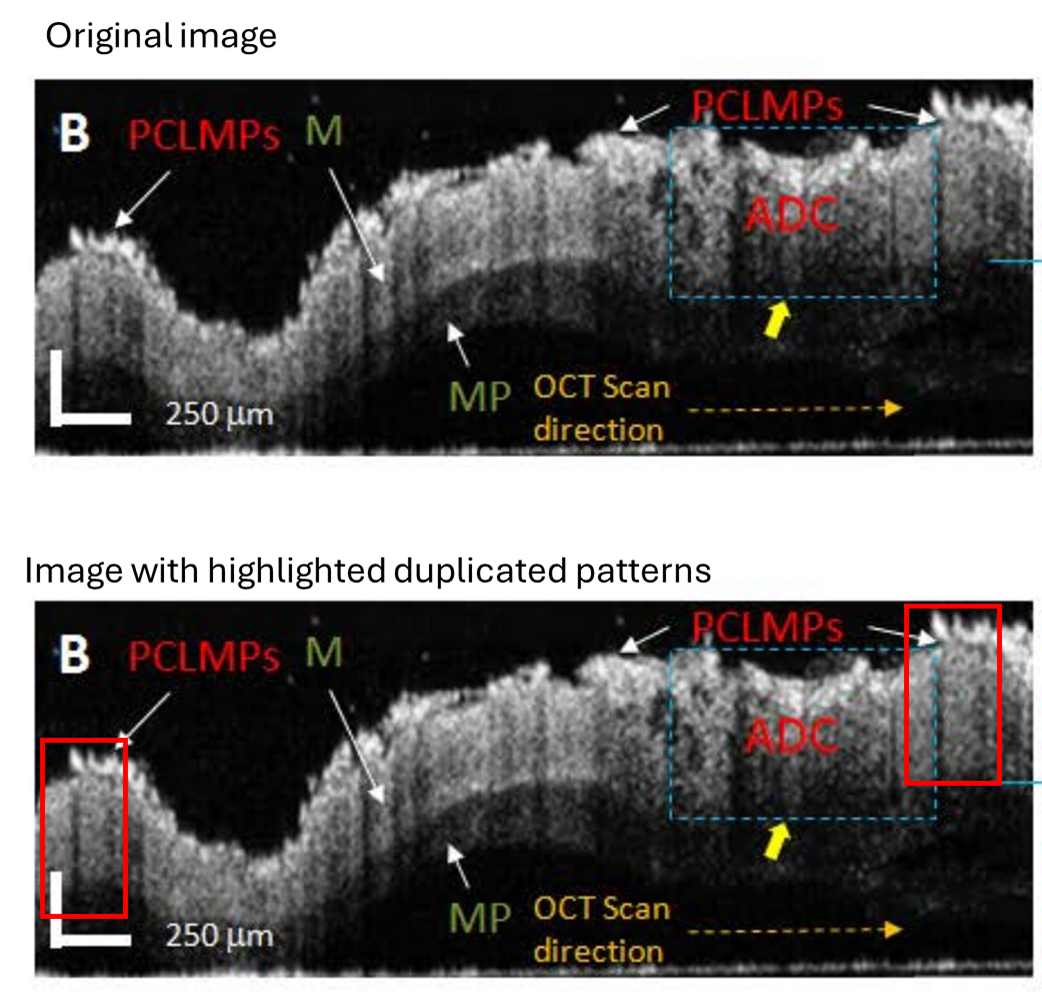
Chesneya tadzhikistana: “Can the authors have a look at FIgure 10B? Two identical patterns can be found within the same OCT image.”
Amiji stated on PubPeer that these “somewhat overlapping features” were “due to specific NPs adhesion to tissue.” His first author Nicusor Iftimia, who manages biomedical optics technologies at a company, advised to ignore things “at the microscale” and suspected “a frame sync issue“, but alas, “the original datafile is no longer available, as it was not archived.” The expert then accused the PubPeer commenters of “tendentious and insulting” behaviour.
While not all of these problems are worth Amiji’s time (only 6 responses on PubPeer, remember?) sometimes talking to one of his co-authors is more helpful. A second corrigendum has hit the polymath tower…. We meet again Amiji’s friend at University of Porto, Bruno Sarmento, president-elect of Controlled Released Society:
Flávia Sousa, Harkiranpreet Kaur Dhaliwal, Florence Gattacceca, Bruno Sarmento, Mansoor M. Amiji Enhanced anti-angiogenic effects of bevacizumab in glioblastoma treatment upon intranasal administration in polymeric nanoparticles Journal of Controlled Release (2019) doi: 10.1016/j.jconrel.2019.07.033
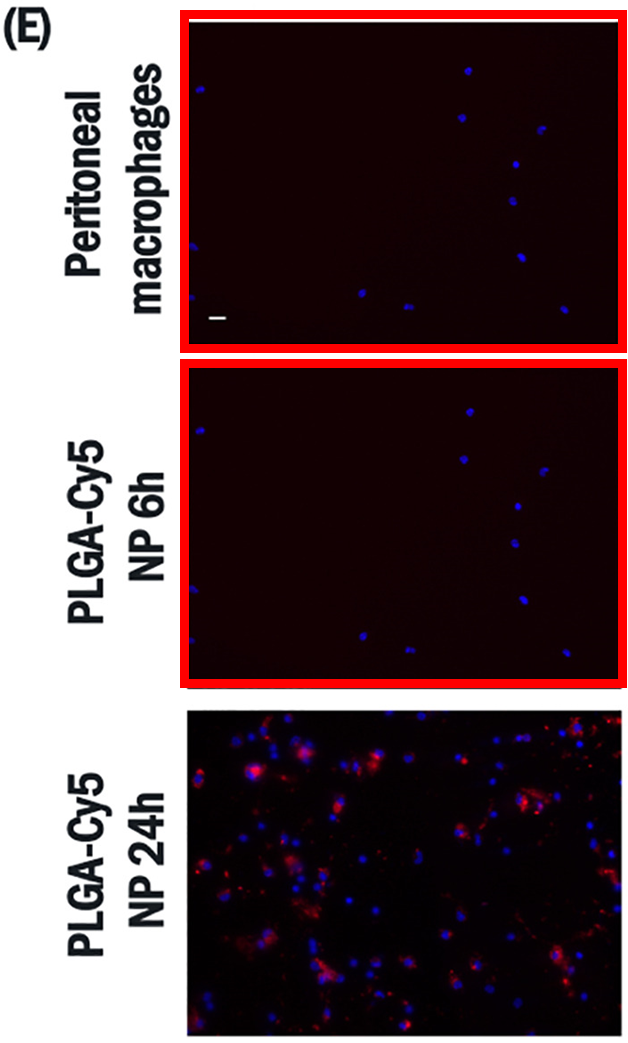
Flaviflexus huanghaiensis: Figure 1E: two images are identical for different conditions
First author Flávia Sousa, now assistant professor at University of Groningen, Netherlands, and a role model for women in science, was quick to respond on PubPeer, and within three months, in October 2025, a Corrigendum was published!
“The authors regret that in the originally published version of this article, an error occurred during the assembly of Fig. 1e. Specifically, the peritoneal macrophages control image was inadvertently misplaced in the process of combining images […]
This correction does not affect the description, interpretation, or conclusions of the study.”
Good thing this doesn’t affect the study or its conclusions at all! Was anything else mentioned on PubPeer that might warrant any concerns here?
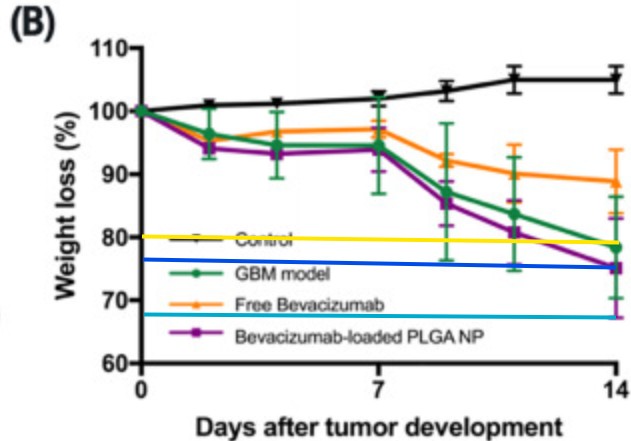
Sousa addressed this already in August 2025, she admitted on PubPeer that “animals approached or briefly exceeded this threshold“, but no worries, she is “reviewing the original raw data and the IACUC-approved protocol” and is otherwise “committed to adhering to the highest standards of ethical research and animal care“.
And then they closed the case with the Corrigendum.
Let’s not focus on a few negatives, let us instead study what other achievements Professor Amiji has under his belt that helped him along the way to becoming Fellow to the National Academy of Inventors?
Just this year he has published 15 articles on various applications on micro and nano-whatever across the human body including (but not limited to) nasal airway epithelial cells, pancreatic cancer, multiple myeloma, acute liver injury, and inflammatory bowel disease. And only four of those publications are corrections!
For example, this review in Sinusitis about “The Effects of Microplastics and Nanoplastics in the Nasal Airway and Upper Respiratory Tract” is right up Amiji’s alley because it has the word “nano” in the title.
Amiji was also a co-PI for a grant regarding “Financial Network Disruptions” surrounding the distribution of counterfeit or illegal medicines. This was a “planning” grant for the Disrupting Operations of Illicit Supply Networks (D-ISN) group to disrupt “illegal medical and pharmaceutical supply chains“. Not sure what this has to do with nanomedicines but Amiji is a “polymath” after all!
Unfortunately, Amiji was not included in the two articles that was credited to this grant, which could have added “online servers” and “magnetic microwire neural networks” to his long list of expertise. Neither of which are overtly relevant to overthrowing the fentanyl crisis in the US illegal medical and pharmaceutical supply chains.
That’s okay, Amiji has over 500 other published articles and 41144 citations according to his Google Scholar profile. It’s important to recognise the friends who have helped him along the way to becoming Fellow to the National Academy of Inventors, such as his former postdocs at Northeastern, Amit Singh (now in pharma industry) and Srinivas Ganta (now at FDA). Some of Amiji’s most cited articles are from his postdoc time with Amiji, featuring a list of hard hitters like “curcumin”, “and “hyaluronic acid”, and other miracle cures for cancer.
They are often featured together on PubPeer, as almost every entry for one includes the others, some of which we’ve already covered.
Here are Amiji and Singh with the Sarmento group:
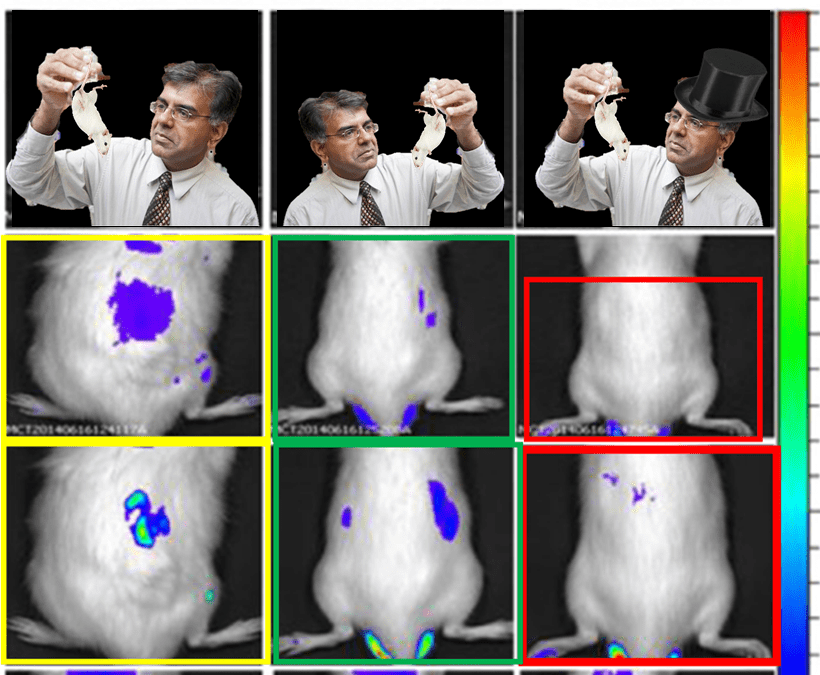
Ana Vanessa Nascimento , Florence Gattacceca , Amit Singh , Hassan Bousbaa , Domingos Ferreira , Bruno Sarmento , Mansoor M Amiji Biodistribution and pharmacokinetics of Mad2 siRNA-loaded EGFR-targeted chitosan nanoparticles in cisplatin sensitive and resistant lung cancer models Nanomedicine (2016) doi: 10.2217/nnm.16.14
Transversotrema cutmorei: “figure 1 of this article, two pair of mouses are “much more similar than expected”.
As someone “fully committed” to upholding “integrity”, Amiji responded the only way he knows how.
“Hello, these images are not identical, as the 2-hour image shows free Cy5 throughout the body, as well as in the liver.”
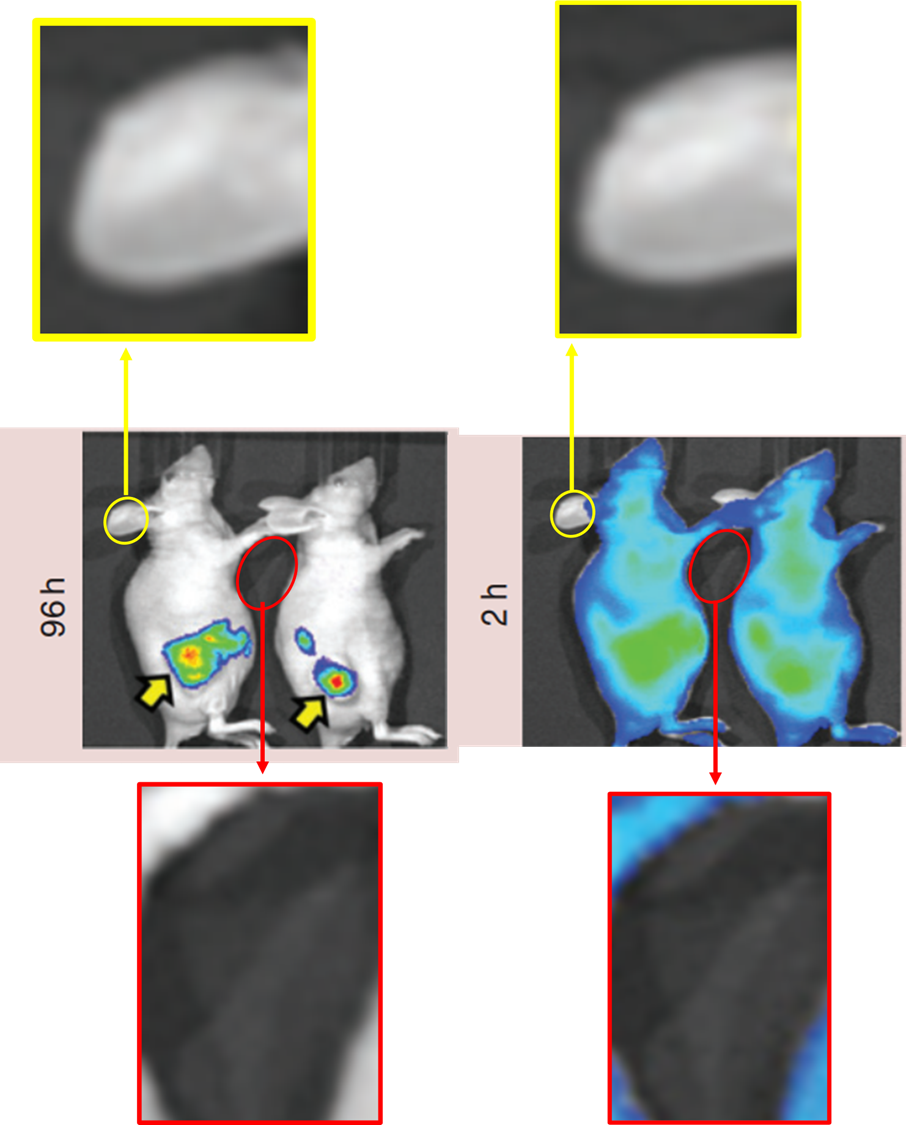
Before ending up at FDA, Ganta used to work for pharma companies, between 2010 and 2013 he was with Nemucore Medical Innovations, which in 2010 started a collaboration with Northeastern University to develop cancer therapies. The company might be close to going defunct, its website doesn’t work properly. The last author on the following paper is the co-founder and CEO of Nemucore, Timothy Coleman:
Srinivas Ganta , Amit Singh , Praveen Kulkarni , Amanda W. Keeler , Aleksandr Piroyan , Rupa R. Sawant , Niravkumar R. Patel , Barbara Davis , Craig Ferris , Sara O’Neal , William Zamboni , Mansoor M. Amiji , Timothy P. Coleman EGFR Targeted Theranostic Nanoemulsion for Image-Guided Ovarian Cancer Therapy Pharmaceutical Research (2015) doi: 10.1007/s11095-015-1660-z
The paper has a section titled “ACKNOWLEDGMENTS AND DISCLOSURES”, yet no disclosures of obvious conflicts of interest are disclosed there. The next study on nanoemulsions for treatment of cancer is even more shameless in this regard:
Srinivas Ganta , Amit Singh , Yashesh Rawal , Joseph Cacaccio , Niravkumar R. Patel , Praveen Kulkarni , Craig F. Ferris , Mansoor M. Amiji , Timothy P. Coleman Formulation development of a novel targeted theranostic nanoemulsion of docetaxel to overcome multidrug resistance in ovarian cancer Drug Delivery (2016) doi: 10.3109/10717544.2014.923068
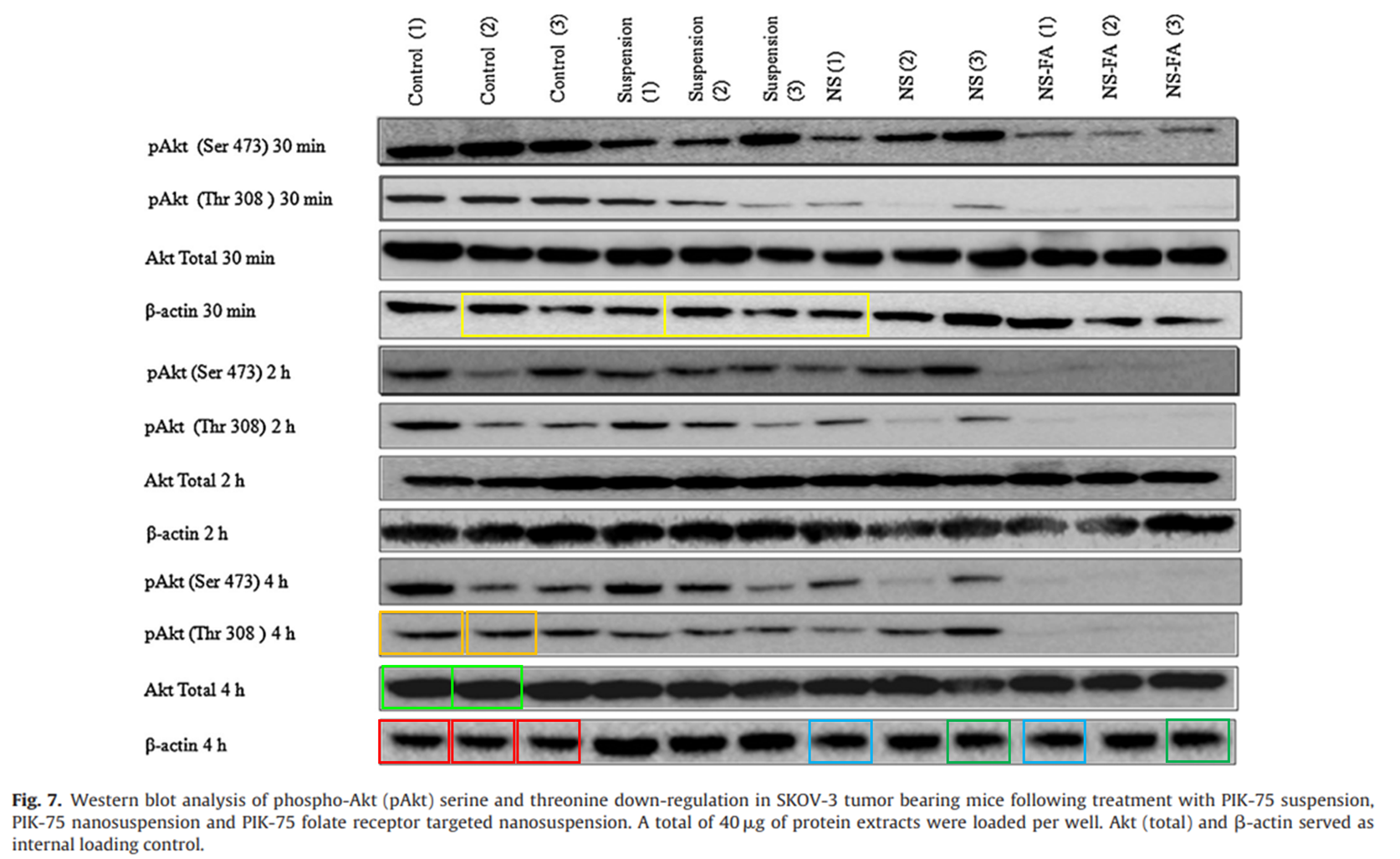
Viola sheltonii: “Can the authors please provide the original western blot image for Figure 7? This shows four [three?] different blots copy and pasted together.”
We are told: “The authors report no conflicts of interest.”
But it is not only Coleman, Ganta and other Nemucore employees who are lying here. Amiji declared in a 2012 interview with the Controlled Release Society (where he is a fellow):
“We are working closely with scientists at Nemucore Medical Innovations, a start-up company that has licensed our nano-emulsion technology. […]
We are moving in this direction by partnering with companies so they can take academic technologies and develop these into clinically viable therapeutics, such as a targeted nanoemulsion formulation with combination therapy for treating refractory ovarian cancer that is being developed by Nemucore.”
Of course Amiji is Nemucore’s co-founder and sits on its advisory board, and on that of other pharma companies, some of which he himself founded, like Perosomer Therapeutics.

You see, honesty was never something Amiji felt comfortable with, my advice is that you don’t rely on him even for the time of the day. He did reply to me eventually:
“Thank you for bringing this very serious matter to my attention. I have been at Northeastern University for 33 years now and I can assure you that we conduct our research with the highest level of ethical integrity and responsibility.
Regarding the PubPeer comment, I have reached out to Drs. Srinivas Ganta and Timothy Coleman (both copied on this email) for further clarification as this publication originated from Nemucore. Regarding the COI statement, I was affiliated with Nemucore in the past, but have divested from the company.“
Funny, how those stop being Amiji’s papers when fraud is found. Anyway, here is another nanoemulsion study, with Ganta’s former mentor in Australia, Sanjay Garg:
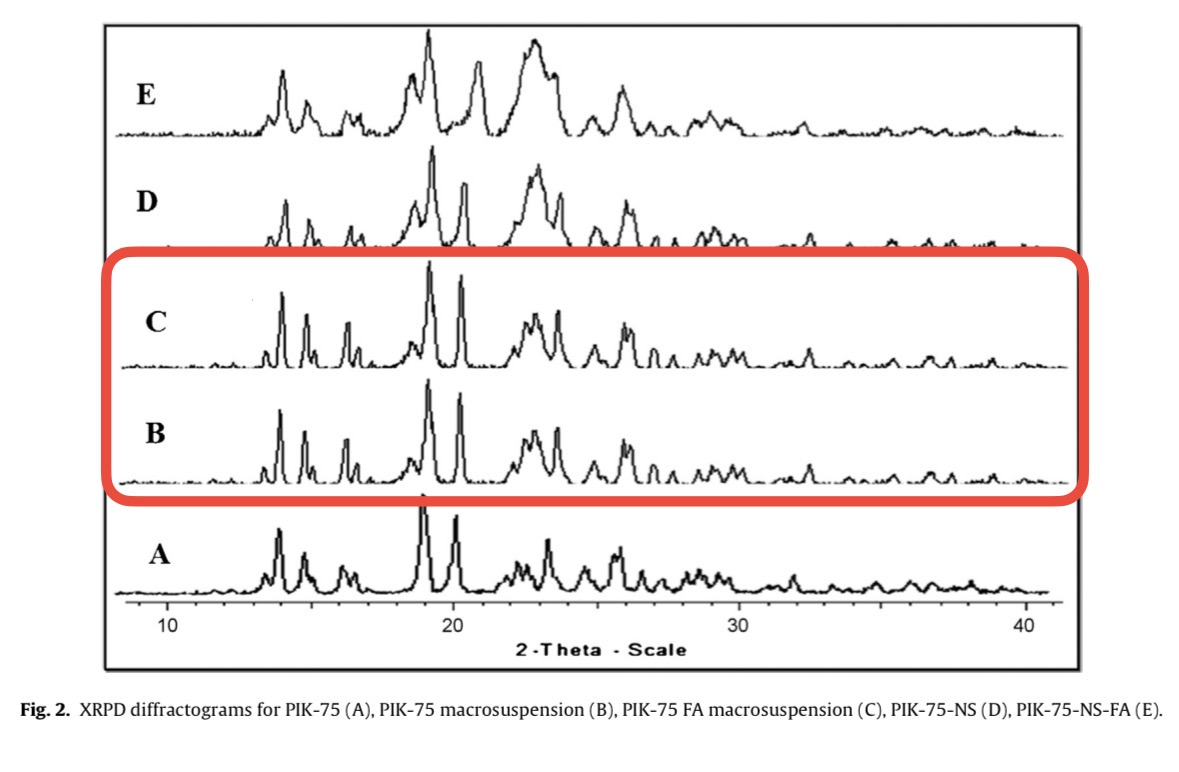
Meghna Talekar , Srinivas Ganta , Mansoor Amiji , Stephen Jamieson , Jackie Kendall , William A. Denny , Sanjay Garg Development of PIK-75 nanosuspension formulation with enhanced delivery efficiency and cytotoxicity for targeted anti-cancer therapy International Journal of Pharmaceutics (2013) doi: 10.1016/j.ijpharm.2013.04.057
Garg is a horrible mouse abuser, see Shaikh et al 2017, and this is what he and Ganta published before:
Srinivas Ganta , James W. Paxton , Bruce C. Baguley , Sanjay Garg Formulation and pharmacokinetic evaluation of an asulacrine nanocrystalline suspension for intravenous delivery International Journal of Pharmaceutics (2009) doi: 10.1016/j.ijpharm.2008.09.022
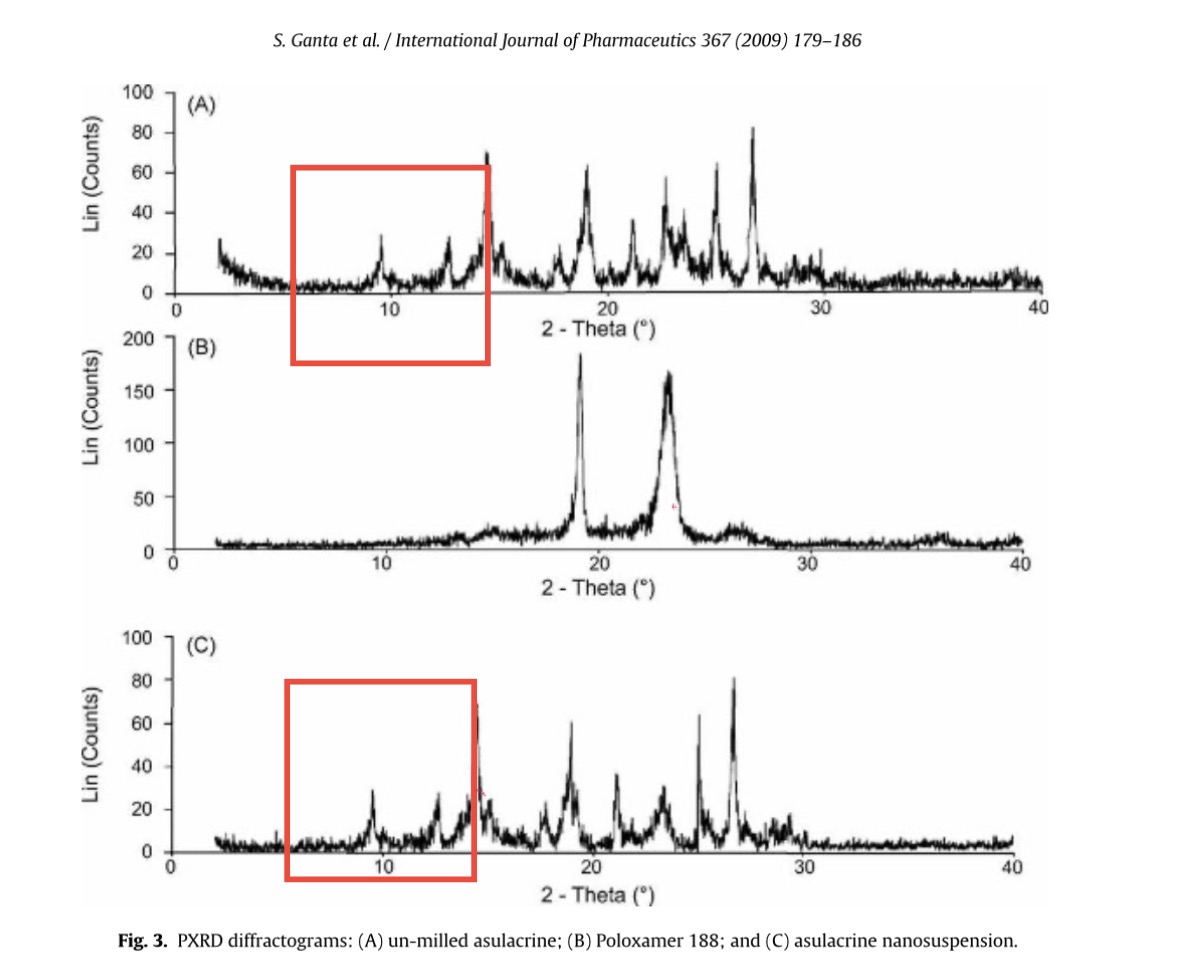
Panicum machrisianum: “Figure 3: a part of one XRPD spectrum is identical to a part of another one”
Ganta continued publishing with Coleman even after he left his and Amiji’s company, see for example this Nemucore-funded study where most authors are from Nemucore:
Niravkumar R. Patel , Aleksandr Piroyan , Srinivas Ganta , Allison B. Morse , Katie M. Candiloro , April L. Solon , Abbegail H. Nack , Corin A. Galati , Collete Bora , Marisa A. Maglaty , Shane W. O’Brien , Samuel Litwin , Barbara Davis , Denise C. Connolly , Timothy P. Coleman In Vitro and In Vivo evaluation of a novel folate-targeted theranostic nanoemulsion of docetaxel for imaging and improved anticancer activity against ovarian cancers Cancer Biology & Therapy (2018) doi: 10.1080/15384047.2017.1395118
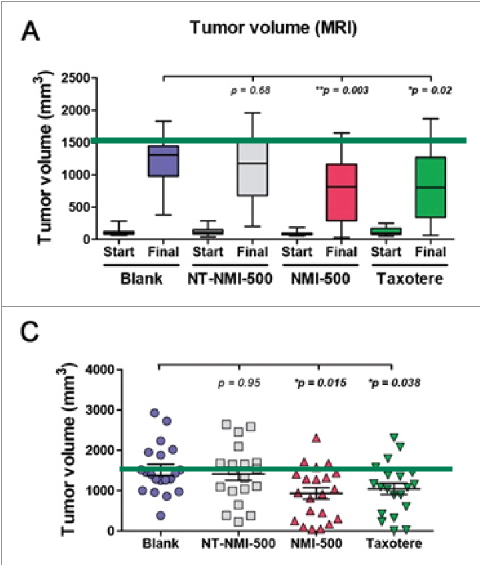
Viola sheltonii: “Figure 5A and 5C show that there are multiple mice from all treatment groups that surpassed the stated humane endpoint of ~1500mm3 (green line added to graphs at 1500mm3), when measured by MRI and further when mice were sacrificed.”
There still was no space for a COI section, but lots of space for animal abuse.
In one rather bizarre case, Ganta, Singh and Amiji stole a figure from a Chinese paper by unrelated authors, and then attributed it to Amiji’s older paper with Langer!
Arun K. Iyer , Amit Singh , Srinivas Ganta , Mansoor M. Amiji Role of integrated cancer nanomedicine in overcoming drug resistance Advanced Drug Delivery Reviews (2013) doi: 10.1016/j.addr.2013.07.012
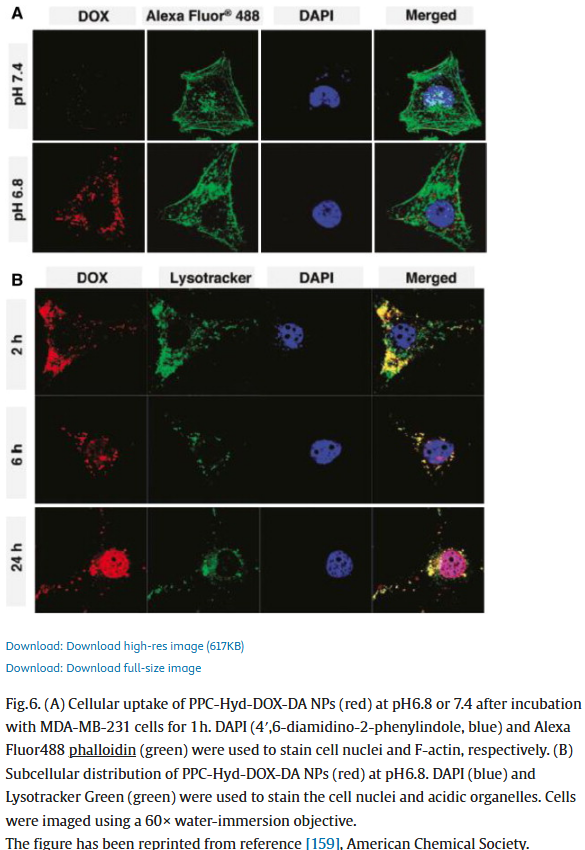

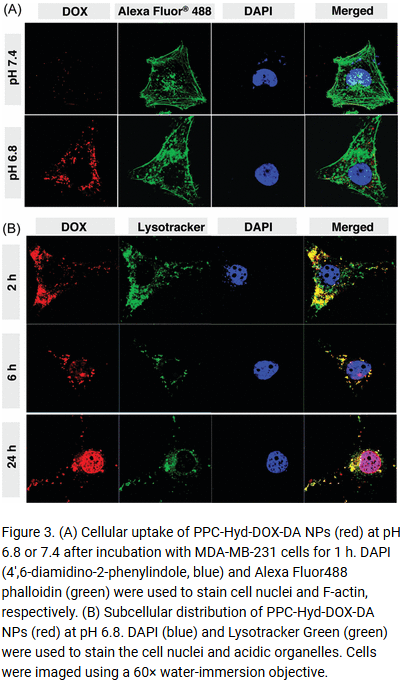
It’s this kind of manufacturing that earns you the title of “inventor”, not just for nano-micro-tiny-small-(other synonym) based drug delivery but also inventing new ways to deny reusing images and manipulating data. This probably also earned Amiji the American Association for the Advancement of Science’s 2024 lifetime fellowship, which Northeastern University posted twice to their Instagram.
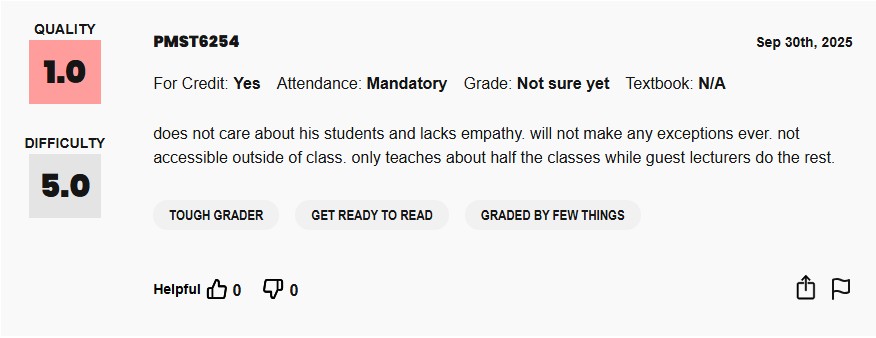
A pinnacle of scientific recognition. That’s definitely what his students think about him too!
The For Better Science Rate My Author gives Amiji a 5/5 for never admitting to duplicating images but only a 1/5 in being “fully committed to upholding the integrity of the scientific record“.
By the way, Amiji did previously get a mention on For Better Science – it was his collaboration with the gangster duo from Miami, Francis Hornicek and Zhenfeng Duan, exposed by Sholto David:
Miami Vice: Francis Hornicek & Zhenfeng Duan
” I will only confirm that every conclusion offered in papers on which I am one of the authors is sound and can be relied upon.” Francis Hornicek
Oh, and another thing. In 2019, Amiji joined other scientists in publishing an authoritative opinion in Nature Nanotechnology, titled: “On the issue of transparency and reproducibility in nanomedicine“. He contributed a section together with Sarmento and other members of the “Nanomedicine and Nanoscale Delivery Focus Group of the Controlled Release Society”.
Their proposed solution to the criss of reproducibility: “faster and effective translation of nanomaterials into the clinics” via “standardization of the terminology“.
We have other, more radical solutions, but those would involve people like Amiji becoming unemployed.
Donate!
If you are interested to support my work, you can leave here a small tip of $5. Or several of small tips, just increase the amount as you like (2x=€10; 5x=€25). Your generous patronage of my journalism will be most appreciated!
€5.00








‘Their proposed solution to the criss of reproducibility: “faster and effective translation of nanomaterials into the clinics” via “standardization of the terminology“.’
When a nanotech researcher claims that they can address the reproducibility crisis primarily by standardizing terminology in order to enable faster and more effective clinical translation, this should raise immediate concern. Reproducibility problems in nanoscience are not driven by linguistic ambiguity but by physical and biological variability, including differences in synthesis conditions, insufficient characterization of size, shape, and surface chemistry, instability over time, and unpredictable interactions with biological systems. Reframing these technical failures as a communication problem allows the researcher and their ventures to appear constructive while avoiding the far more difficult task of demonstrating that their materials behave reliably.
A particularly telling red flag is the promise of faster clinical translation without corresponding evidence of validation. Translation slows down because nanomaterials frequently fail when moving from one laboratory to another, or from in vitro to in vivo systems. If a nanotech researcher who founds startups cannot point to independent replication, inter-laboratory studies, reference materials, or sustained performance across batches, claims of speed amount to marketing language rather than scientific progress. Standardized words do not reduce biological variability or experimental noise, and they certainly do not address fraud.
Another warning sign is the substitution of process narratives for experimental proof. Researcher-founded startups with weak or irreproducible data often emphasize frameworks, ontologies, working groups, or white papers, while offering little access to raw data, negative results, or failed experiments. Process reform is inexpensive and difficult to falsify, whereas reproducible nanomaterials are costly and unforgiving. This imbalance suggests that the emphasis on terminology serves as a shield against deeper scrutiny.
The way standardization itself is used also matters. Genuine standards specify exactly which parameters are controlled, how they are measured, who enforces compliance, and how deviations are handled. When standardization is described vaguely, without naming concrete physicochemical properties, validation methods, or enforcement mechanisms, it functions more as branding than as quality control. The absence of certified reference materials or cross-laboratory calibration further undermines any serious claim to reproducibility.
Broad claims that a single terminology framework can accelerate translation across fundamentally different classes of nanomaterials are another indicator of superficial understanding. Lipid nanoparticles, polymer systems, metal oxides, and carbon-based materials fail for different reasons and interact with biology in distinct ways. Treating them as variations of the same problem suggests that the researcher is operating at the level of abstraction rather than mechanism.
Finally, the narrative often shifts responsibility away from the researcher and their ventures. Failures are attributed to field-wide confusion, lack of shared language, or overly cautious regulators, instead of to an inability to demonstrate consistent performance. Without clear, quantitative metrics showing improved reproducibility or predictive power, such claims cannot be meaningfully evaluated. In this context, the emphasis on terminology standardization appears less like a solution to the reproducibility crisis and more like a way to postpone accountability while maintaining a persuasive story for investors.
LikeLike