Schneider Shorts of 13 December 2024 – anti-aging scammers report pushy competitor to the media, an embattled biotech gets its former executive back after lawsuit, plus a bunch of retractions including five for Sicilian ex-rector, and two for already corrected papers by Israeli Scientists.
Table of Discontent
Industry Giants
- David Sinclair’s seductive notion – anti-aging scam competitors complained to WSJ
- Most lying, deceiving person in the world – why Richard Pestell returns to CytoDyn
Retraction Watchdogging
- Fraud against the State and embezzlement – 5 retractions and an expression of concern for Salvatore Cuzzocrea
- Accidentally misused – one retraction and one correction for Anna Bagnato
- No point for “fabrication” of any form – two retractions for 2 corrected papers by Ruth Gabizon
- Concerns regarding the veracity – oil drilling research in Saudi Arabia, hand-made
Industry Giants
David Sinclair’s seductive notion
After years and decades of being celebrated as medical genius and humanity’s greatest hope to overcome aging and death, the Harvard professor David Sinclair is now in free-fall. His reputation is entrepreneur is now in tatters, and who knows, maybe even his fake papers will be retracted one day.
Never-ageing Anti-aging to cure COVID-19
Scientists David Sinclair and Michael Lisanti have an ingenious solution to COVID-19: anti-aging drugs. If a disease kills old people, stop being old!
It seemed, the trouble started when Sinclair broke with some unwritten rules and opened a competing dog-rejuvenation business (Animal Biosciences, Inc), which led to a lot of howling, fist-shaking, foot-stomping and finger-pointing in the anti-aging scammer community, Sinclair was even removed as President of Academy for Health and Lifespan Research (read March 2024 Shorts).
I suspect the anti-aging market is run like the drug and pimping operations by the mafia, with clearly defined areas of influence for each clan to avoid shoot-ups.
The 55 year old Sinclair broke too many such gentlemen agreements by moving into the cornered businesses of his academic peers. Rich pampered boys in professor chairs hyperventilated and ran to complain to the media. This is the result, in The Wall Street Journal, from 5 December 2024:
“Harvard geneticist David Sinclair’s seductive notion that aging is a treatable disease has helped companies he founded to raise more than $1 billion. The investors have almost nothing to show for it.
Four companies trying to develop longevity drugs have gone bankrupt or largely halted operations. Another four either haven’t yet tested their drugs or gene therapies in humans or have run only small-scale trials that make it difficult to know whether a drug will work. […]
Sinclair also has co-founded companies that sell directly to consumers products such as antiaging dog chews, supplements and tests that purport to show one’s “biological age.”
That’s a clue dear reader, that one of those who ran to complain about Sinclair to journalists must be Steve Horvath who sells his own “Horvath Clock” tests for “biological age” (read September 2024 Shorts).
The original sins of Leonard Guarente
“Without specific and credible allegations of research misconduct, MIT is unable to take any action.”
WSJ narrates a story of Sinclair’s anti-aging scams:
“Sinclair’s business career began in 2004 when he co-founded Sirtris Pharmaceuticals. His lab found that resveratrol, a compound found in red wine, appeared to prolong lifespan in worms, yeast and other organisms. Sirtris wanted to make a reformulated version. He was among the first to get a big pharmaceutical company to buy into the notion that a longevity drug could get to market, selling Sirtris in 2008 to GSK—then GlaxoSmithKline—for $720 million. His shares at the time were worth $9.3 million. […] In 2010, GSK stopped testing the drug in cancer patients over safety concerns. Three years later, GSK announced it was closing Sirtris.”
Next, Sinclair decided to get some older women pregnant:
“In 2011, Sinclair and colleagues from Sirtris founded OvaScience to commercialize research they believed would help older women become pregnant. It went public in 2012. […] The stock peaked in March 2015, at $53, which valued the company at more than $1.3 billion. Sinclair’s stake of more than 700,000 shares was worth some $37 million at the time, according to the Journal’s analysis of regulatory filings.
Later that month, OvaScience said its treatment hadn’t improved IVF success rates in patients. Its shares tumbled 35%, to $34.73. By March of 2018, the stock was trading for less than $1. “
Sinclair’s next business is the obesity market:
“Around the time OvaScience’s stock was peaking in 2015, another company Sinclair co-founded, CohBar, was going public to develop treatments for obesity and fatty liver disease. The founders raised nearly $75 million overall. […]
In 2022 it suspended development on its leading drug candidates, citing safety concerns and disappointing study results. Shareholders sued CohBar in 2023, saying they had been misled about a merger CohBar planned with oncology company Morphogenesis. The merger fell apart that year and CohBar said it would dissolve. “
The Island of Dr Izpisua Belmonte
Human-monkey chimeras arrive to solve the problem of organ shortage. Thank Juan Carlos Izpisua Belmonte, who is ready to cure all possible diseases and even the old age. With chutzpah and Cell on his side.
CohBar co-founder, the Israeli Nir Barzilai of Albert Einstein College of Medicine in New York, claims to have discovered the “longevity gene” and since many years pushes the diabetes metformin as anti-aging miracle drug. He isn’t a friend of Sinclair’s anymore. Years ago, Barzilai introduced Sinclair to WeWork CEO Adam Neumann, who gave Sinclair $25 million to start another company:
“In 2017, Sinclair co-founded Life Biosciences, a longevity incubator set up to turn promising findings from scientists’ labs into startups. […] Over the years, it raised about $158 million. It licensed a gene therapy from Sinclair’s Harvard lab to develop as a possible treatment for a rare eye condition that causes sudden vision loss. […] Life Biosciences closed subsidiaries, cut its workforce to 13, shut its lab and contracted most work on the therapy to outside researchers”
Sinclair’s Life Biosciences tortured many monkeys, having first blinded them with lasers, then subjected them to “gene therapy”, then claimed in last year’s press releases that the monkeys could partially see again. A clinical trial on humans is scheduled for 2026.
George Church, Colossal W*nker
From mammoths to eugenics to anti-aging scams: god-impersonator George Church knows how to make money with bullshit.
Sinclair’s PubPeer record is awe-inspiring. In this regard, allow me to show you what bazilai’s own science occasionally looks like (more on PubPeer):
Ying Lin , Michael W. Rajala , Joel P. Berger , David E. Moller , Nir Barzilai, Philipp E. Scherer Hyperglycemia-induced production of acute phase reactants in adipose tissue Journal of Biological Chemistry (2001) doi: 10.1074/jbc.m107101200


These days, Sinclair is into selling supplements and other crap for bored rich ageing gits:
“Metro International Biotech, which Sinclair helped found in 2016, is trying to develop a formulation of nicotinamide mononucleotide, or NMN, as an FDA-approved drug to treat age-related conditions such as Alzheimer’s disease.” […]
In 2022, he and celebrity chef Serena Poon, who he describes as both his personal partner and business partner, started Fully Aligned, which calls itself a consumer wellness company. […]
He co-founded Tally Health in 2022 with Whitney Casey, a partner at a private-equity firm. Casey got interested in the Sinclair lab’s efforts to develop a cheek-swab test to tell people their biological age, a measure of how fast their body is aging physically rather than chronologically. Tally started selling $229 test kits and touting celebrity investors…”
I guess Sinclair’s barging into the NMN supplement and “aging clock” market which is already controlled by his former mentor Leonard Guarente and his Nobelist-studded Elysium Health, wasn’t met with applause.
Most lying, deceiving person in the world
Richard Pestell, former bigwig at Thomas Jefferson University and author of masses of obviously falsified research papers, may be a bad scientist but he is a genius in making huge amounts of money with dodgy cancer cures.
The Pestilence of Pestell
Richard Pestell MB, BS, MD, PhD, FACP, FRACP. MBA, FRCP, FRSB, AO is the most dashing doctor a girl or a boy can ever dream of. What luck for Michael Lisanti to have been invited for a ride!
The biotech company CytoDyn announced on 25 November 2024 to have appointed Pestell as Lead Consultant in Oncology:
“He previously served as Vice Chairman of the Board, and Chief Medical Officer (CMO), spearheading the Company’s successful effort to obtain Fast Track Designation from the FDA for the use of leronlimab in combination with carboplatin for the treatment of patients with CCR5-positive metastatic triple-negative breast cancer. In addition, Dr. Pestell was instrumental in designing and initiating CytoDyn’s Phase 1b/2 clinical trial in that indication. […]
Dr. Pestell added, “I am confident that leronlimab holds significant promise for improving patient care and expanding treatment options in oncology. I am eager to support the Company’s efforts to advance our clinical development pipeline and leverage the potential of leronlimab to improve outcomes for cancer patients.””
Pestell (who originally joined CytoDyn in January 2019 having sold to them his company ProstaGene), is definitely the right person to push Cytodyn’s drug leronlimab, which is a CCR5-receptor antibody. This time for cancer, because COVID-19 is off the menu, as Geekwire reported in January 2022:
“Nader Pourhassan has been ousted as CEO and president by the board of directors of embattled Vancouver, Wash.-based CytoDyn […] Pourhassan’s termination comes as the company faces investigations from the U.S. Securities and Exchange Commission and the U.S. Department of Justice. The company was also strongly rebuked by the U.S. Food and Drug Administration in May.
The FDA chastised the company for its claims about an experimental drug, leronlimab, which it tested in a failed clinical trial for COVID-19. The rebuke was followed by subpoenas from the SEC and the DOJ for documents related to company statements about the use of leronlimab in HIV, cancer, and COVID-19, as part of ongoing investigations.”
Pourhassan was paid whopping $10 million per year as CEO. The article continues:
“And if that was not enough, the company also last year faced lawsuits from shareholders and a failed attempt at a board takeover by activist shareholders. The takeover bid provided an even weirder twist to CytoDyn’s year. The takeover attempt was led in part by former CytoDyn advisor Bruce Patterson, a physician and CEO of diagnostics company IncellDx. Patterson is also affiliated with the Front Line Covid-19 Critical Care Alliance, which endorses the unproven drug ivermectin to treat COVID-19 in protocols he helped develop.”
As it happens, I wrote about Bruce Patterson and his IncellDx and his phony claim to cure Long COVID with the approved HIV drug maraviroc (a CCR5 receptor antagonist) in August 2022 Shorts. This is the FDA statement and this is that TED Talk by Patterson which caused it:
Please refer to Mother Jones reporting from January 2022 for more details, quote:
“IncellDX announced the details of one of its trial sites. The lead physician is a Milwaukee-based oral surgeon, holistic medicine practitioner, and anti-aging doctor. Plus, IncellDX isn’t waiting for the results of the trial to embark on new projects. The team has begun to offer its treatment for several other illnesses, including chronic Lyme disease and Myalgic Encephalomyelitis/Chronic Fatigue Syndrome. It’s also expanding its services to Europe, and it’s launching a program for children with long Covid.”
Here is Patterson’s relevant paper in Scientific Reports from August 2024, titled “Long COVID diagnostic with differentiation from chronic lyme disease using machine learning and cytokine hubs“.
Christian Perronne and other Chronic Lymericks
France is enthralled by the charm of Professor Christian Perronne. With COVID-19 almost vanquished by chloroquine magic, what about the Chronic Lyme Disease? Smut Clyde goes where even Didier Raoult refused to go.
Having failed to take over CytoDyn and its leronimab, Patterson returned to pushing maraviroc. In February 2024, his company HealthBioAI (where InCellDx has been incorporated) received a permission from FDA to proceed with a clinical trial for maraviroc and a statin on 252 Long COVID patients.
Now remember Cytodyn’s past takeover troubles and their new oncology head. In August 2021, BioSpace reported:
“CytoDyn is fighting back and has filed a lawsuit against the activist group led by Paul Rosenbaum and Bruce Patterson, alleging that they are misleading shareholders and “waging an illegal proxy contest to take over control of the Company’s Board of Directors. […] CytoDyn says that two of the activists include former directors: former chief medical officer Richard G. Pestell, who was fired for cause and who then sued the company; and former executive board chair Anthony D. Caracciolo.”

In fact, CytoDyn had to re-employ Pestell as “a non-cash settlement“, as Deep Dive reported in May 2024:
“The settlement entails a quitclaim from both parties. The company is also giving Dr. Pestell 8.3 million company shares and a purchase warrant for 7.0 million more shares at $0.37 per share. Dr. Pestell then filed a breach of contract and defamation lawsuit against the company, its former CEO Dr. Nader Pourhassan, and Kelly. In the suit, he claims that he is entitled to “separation obligations” since he was fired. He also claimed that Dr. Pourhassan “embarked on an effort to retaliate” when Dr. Pestell raised safety concerns related to the firm’s US FDA drug application.
Dr. Pourhassan responded, saying Dr. Pestell is the “most lying, deceiving person in the world.””
Back in May 2024, CytoDyn stock traded at $0.33. Now with Pestell back, Cytodyn trades at $0.11.
Retraction Watchdogging
Fraud against the State and embezzlement
Salvatore Cuzzocrea, the ex-rector of the University of Messina, continues to lose papers. There are five new retractions, 3 in Journal of Pineal Research and 2 in Immunity, both by Wiley. By now Cuzzo’s retraction count should stand at 14.
Cuzzocrea’s Magnificent Fall
“These unscrupulous charlatans in Messina should be fired on the spot tomorrow morning, forced to return twenty years of undeserved wages and sent to work the land” – Aneurus Inconstans
Let’s start with this one:
Roberta D’Emmanuele Di Villa Bianca, Stefania Marzocco , Di Paola Rosanna, Giuseppina Autore , Aldo Pinto , Salvatore Cuzzocrea , Raffaella Sorrentino Melatonin prevents lipopolysaccharide‐induced hyporeactivity in rat Journal of Pineal Research (2004) doi: 10.1046/j.1600-079x.2003.00111.x

Aneurus inconstans: “Figure 6A and 8A: these two micrographs overlap (red boxes), but they are supposed to show immuno-localizaion of either iNOS or nitrotyrosine, respectively, in Sham-operated rats.”
The retraction from 29 November 2024 went:
“The retraction has been agreed due to concerns raised by third parties on the data presented in the article. Specifically, there is evidence of image duplication between Figures 6A and 8A, indicating that the same biological sample has been utilized to represent two distinct experimental conditions. The article is retracted as the editors have lost trust in the accuracy and integrity of the overall body of data presented in the article and consider its conclusions invalid.”
Next, a 25 year old paper coauthored by Messina’s (now emeritus) professor Achille Caputi:
Salvatore Cuzzocrea, Giuseppina Costantino , Emanuela Mazzon , Achille P. Caputi Regulation of prostaglandin production in carrageenan‐induced pleurisy melatonin Journal of Pineal Research (1999) doi: 10.1111/j.1600-079x.1999.tb00591.x

Aneurus inconstans: “Figure 3: COX-2 staining in the lungs of carrageenan-treated rats. The same image (rotated by 90 degrees) was published at the same time by the same people in Figure 4 of Cuzzocrea et al. 1999 Eur J pharmacol, where it is described as nitrotyrosine staining in the lungs of carrageenan-treated rats pretreated with BSO (A).”
Here the similarly-worded retraction notice from 29 November 2024 mentioned
“…the sample shown in Figure 3A has been identified as being used in a different scientific context in another article from the same author group, which was submitted concurrently. The data provided by the corresponding author upon request was insufficient to address the concerns.”
Now a 26 year old paper which features Cuzzocrea’s close associate and Sicilian native Basilia Zingarelli, professor and basic research director at Cincinnati Children’s Hospital in USA. As past president of the Shock society, Zingarelli makes sure that none of Cuzzocrea’s many fraudulent papers gets retracted in the society journal Shock.
Salvatore Cuzzocrea, Basilia Zingarelli , Giuseppina Costantino , Achille R Caputi Protective effect of melatonin in a non‐septic shock model induced by zymosan in the rat Journal of Pineal Research (1998) doi: 10.1111/j.1600-079x.1998.tb00382.x

Aneurus inconstans: “Figure 6, caption states: “Effect of melatonin treatment on zymosan-induced lung injury. A: Sham rats […]. C: Lung section from a zymosan-treated rat that received melatonin“
The retraction from 29 November 2024 mentioned “evidence of image duplication between Figures 6A and 6C, indicating that the same biological sample has been utilized to represent two distinct experimental conditions.”
Now to the other two retractions. This paper by Cuzzocrea, Caputi and Zingarelli was also 26 years old:
S Cuzzocrea, A P Caputi, B Zingarelli Peroxynitrite-mediated DNA strand breakage activates poly (ADP-ribose) synthetase and causes cellular energy depletion in carrageenan-induced pleurisy Immunology (1998) doi: 10.1046/j.1365-2567.1998.00409.x

On 5 December 2024, it was retracted:
“The retraction has been agreed due to concerns raised by third parties on the data presented in the article. Specifically, the Western Blot experiment in Figure 2 was found to have been presented in multiple publications with at least one common author and in a different scientific context. The article is retracted as the editors have lost trust in the accuracy and integrity of the overall body of data presented in the article and consider its conclusions invalid. No confirmation of the decision of retraction could be obtained by the authors.”
The next retraction, in the same Wiley journal, was a 21 year old paper, Caputi is again coauthor:
Salvatore Cuzzocrea, Antonietta Rossi , Ivana Serraino , Rosanna Di Paola, Laura Dugo, Tiziana Genovese , Domenico Britti , Giuseppe Sciarra , Angelina De Sarro , Achille P. Caputi, Lidia Sautebin 5‐lipoxygenase knockout mice exhibit a resistance to acute pancreatitis induced by cerulein Immunology (2003) doi: 10.1046/j.1365-2567.2003.01715.x

The same image (blue boxes) appears in Figure 2B of Cuzzocrea et al. 2002 Mol Pharmacol, a paper published a year earlier by the same group, where the same micrograph allegedly describes the immunolocalization of PARP after 15d-PGJ2 treatment.”

The same image rotated and rescaled (red boxes) appears in Figure 1B of Cuzzocrea et al. 2002 Mol Pharmacol, where it’s described as immunohistochemical localization of COX-2 in the lung of 4 hr carrageenan treated rats, but also appears in Figures 4 and 5 of of Cuzzocrea et al. 2002 Biochem Pharmacol, where it is inconsistently described as immunohistological localization of ICAM-1 and P-selectin under yet other different pre-treatments.”

The retraction from 5 December 2024 was similar, mentioning that “two panels of Figure 9 were found to have been previously published in articles with at least one common author and presented in a different scientific context.”
Queen Mary and John Vane’s Cowboys
Welcome to the the William Harvey Research Institute in London. Meet two proteges of its founder, the late Nobelist Sir John Vane: Chris Thiemermann and Mauro Perretti. Then meet their own rotten mentees, especially Salvatore Cuzzocrea and Jesmond Dalli.
This Expression of Concern, in another Wiley journal, is rather interesting though. Cuzzocrea’s and Caputi’s coauthor is his former mentor Christoph Thiemermann, institute director at Queen Mary University London:
Salvatore Cuzzocrea, Barbara Pisano , Laura Dugo, Angela Ianaro , Nimesh S A Patel , Rosanna Di Paola, Tiziana Genovese , Prabal K Chatterjee, Massimo Di Rosa , Achille P Caputi, Christoph Thiemermann Rosiglitazone and 15‐deoxy‐Δ12,14‐prostaglandin J2, ligands of the peroxisome proliferator‐activated receptor‐γ (PPAR‐γ), reduce ischaemia/reperfusion injury of the gut British Journal of Pharmacology (2003) doi: 10.1038/sj.bjp.0705419



Now, those are classical “corner clones” which arise when the original figure labelling in panels’ corners was done in conflict with the journal’s typesetting standards. Thus, patches from somewhere inside image are used to cover up the old corner labels, and new labels are placed. Even in the sleuth community, it is believed that the publishers used to do such inappropriate editing themselves after the manuscript acceptance, and there were indeed cases where publishers admitted it, read here:
Thomas Südhof and AI-powered weapons of micro-duplication
Thomas Südhof, victim of racist and sexist persecution, announced to retract a second paper, “even though the quantitative analyses and conclusions are correct”.
But not in the above case of Cuzzocrea, Caputi and Thiemermann, this was the Expression of Concern from 2 December 2024:
“The expression of concern has been agreed due to third-party concerns related to the data presented in the article. Indicators for cloned image elements and inappropriate undeclared image modification were found in multiple image parts and several panels in Figures 1, 9, and 11. Due to the significant time elapsed since publication, the authors were unable to provide the original images. Therefore, the journal has decided to issue an Expression of Concern to inform and alert the readers.”
Here the blame for the “corner clones” is placed exclusively with the authors. Which supports my theory that corner clones arise when authors never had the original images, only those stolen elsewhere. When the publishers asks them to change the label formatting, the authors can’t comply. This is when they photoshop those corner clones, although I admit there may be cases where indeed publishers do it for the authors. But in both cases, the original images were never available, and corner clones should in my view always be considered as evidence of fraud.
Meanwhile, the criminal investigation of Cuzzocrea’s in Italy was extended by another 6 months, as local media reported on 26 November 2024 (translated):
“There are four types of cases, namely the now terminated abuse of office, auction rigging, fraud against the State and embezzlement.
The time span that the Prosecutor’s Office has been examining in recent months, including with a series of interrogations of people with the knowledge of the facts, especially among the professors and employees of the University rectorate, is very vast. Apparently the focused dates are around October 4, 2023, and then in a period between 2019 and June 2023.“
Accidentally misused
Elsewhere in Italy, the Top Italian Scientist Anna Bagnato, unit director at the Regina Elena National Cancer Institute in Rome, suffered one more retraction. I reported about this and other of her ridiculously fraudulent papers in August 2024 Shorts. Bagnato has around 40 of them flagged on PubPeer, only 2 were previously retracted.
Now also this retraction, Bagnato is the corresponding author, her coauthors are Bagnato’s former mentor at Regina Elena Institute, Pier Giorgio Natali (now CEO of Janus Pharma) and her close associate Laura Rosanò (now CNR Research Director at Sapienza University of Rome):
Francesca Spinella , Laura Rosanò, Martina Del Duca , Valeriana Di Castro , Maria Rita Nicotra , Pier Giorgio Natali , Anna Bagnato Endothelin-1 inhibits prolyl hydroxylase domain 2 to activate hypoxia-inducible factor-1alpha in melanoma cells PLoS ONE (2010) doi: 10.1371/journal.pone.0011241





The retraction was issued on 5 December 2024:
“After this article [1] was published, concerns were raised about Figs 1–5. Specifically,
- – There appear to be similarities between bands within numerous panels presented in:
- Figs 1B and 1D
- Fig 2A
- Fig 3A
- Fig 4B
- Fig 5A
- – There appear to be similarities between bands between numerous panels presented in:
- Fig 1A and Fig 1B
- Fig 1A and Fig 3C
- Fig 3A and Fig 3C
- Fig 3A and Fig 4A
- – There appear to be irregularities in the background directly surrounding the band in lane 2 of the Fig 4B HIF-1α panel.
The corresponding author disagreed with the journal’s observations but did not provide the underlying raw image data. PLOS remains concerned that the areas remain more similar than would be expected from independent results which, in the absence of the underlying data, cannot be resolved.
In light of the above concerns, which question the reliability and integrity of the reported results, the PLOS ONE Editors retract this article.
AB did not agree with the retraction and stands by the article’s findings. FS, LR, MDD, VDC, MRN, and PGN either did not respond directly or could not be reached.”
The fraud in Bagnato’s and Rosano’s papers was known since around a decade. But until this year, Bagnato and her colleagues never suffered any consequences whatsoever. Hence more recent fake stuff:
- Roberta Cianfrocca , Piera Tocci , Laura Rosanò , Valentina Caprara , Rosanna Sestito , Valeriana Di Castro , Anna Bagnato Nuclear β-arrestin1 is a critical cofactor of hypoxia-inducible factor-1α signaling in endothelin-1-induced ovarian tumor progression Oncotarget (2016) doi: 10.18632/oncotarget.7461
- Lidia Chellini , Valentina Caprara , Francesca Spadaro , Rosanna Sestito , Anna Bagnato , Laura Rosanò Regulation of extracellular matrix degradation and metastatic spread by IQGAP1 through endothelin-1 receptor signalling in ovarian cancer Matrix biology (2019) doi: 10.1016/j.matbio.2018.10.005

And these two papers from last year:
- Ilenia Masi , Valentina Caprara , Francesca Spadaro , Lidia Chellini , Rosanna Sestito , Andrea Zancla , Alberto Rainer , Anna Bagnato , Laura Rosanò Endothelin-1 drives invadopodia and interaction with mesothelial cells through ILK Cell Reports (2021) doi: 10.1016/j.celrep.2021.108800
- Ilenia Masi , Flavia Ottavi , Danila Del Rio , Valentina Caprara , Cristina Vastarelli , Sara Maria Giannitelli , Giulia Fianco , Pamela Mozetic , Marianna Buttarelli , Gabriella Ferrandina , Giovanni Scambia , Daniela Gallo , Alberto Rainer , Anna Bagnato , Francesca Spadaro , Laura Rosanò The interaction of β-arrestin1 with talin1 driven by endothelin A receptor as a feature of α5β1 integrin activation in high-grade serous ovarian cancer Cell Death & Disease (2023) doi: 10.1038/s41419-023-05612-7

The editors of the latter journal (a bunch of crooks around Gerry Melino and Guido Kroemer) swiftly helped Bagnato with a “Author Correction” in October 2024:
“The authors regret that there is a mistake in Fig. 8B as published in the original article. In the first published version of this manuscript, the bioluminescent image of intraperitoneally (i.p) SKOV3-Luc-injected mice for AMB group was accidentally misused during the assembly of the figures. We greatly apologize for this error and are now providing a corrected version of the figure (see new Fig. 8B). The scientific conclusions of our study are not affected by this inadvertent error.”
Cell Death and Depravity
Is the journal Cell Death and Disease a disease itself, parasitised by Chinese paper mills? Can it be cured? Not with this team of doctors on editorial board.
And why shouldn’t Bagnato continue, when there is an entire Nature-family journal, Cell Death & Depravity, by Italian fraudsters for Italian fraudsters! More in the same outlet, waiting to be corrected for unaffected conclusions:
Piera Tocci , Celia Roman , Rosanna Sestito , Valeriana Di Castro , Andrea Sacconi , Ivan Molineris , Francesca Paolini , Mariantonia Carosi , Giovanni Tonon , Giovanni Blandino , Anna Bagnato Targeting tumor-stroma communication by blocking endothelin-1 receptors sensitizes high-grade serous ovarian cancer to PARP inhibition Cell Death & Disease (2023) doi: 10.1038/s41419-022-05538-6

No point for “fabrication” of any form
We remain at PLOS. Ruth Gabizon, professor at the Hadassah Medical School of the Hebrew University of Jerusalem and a promenade supplement pusher, loses two papers in PLOS Pathogens. I reported about her problematic science in May 2023 Shorts.
This is the first retraction, the coauthor Ronen Gabizon is likely Ruth Gabizon’s son, he was at that time PhD student of Assaf Friedler, who in turn is the Dean of the science faculty at Hebrew University, and who was supposed to be investigating Gabizon’s PubPeer record, yet decided not to, for obvious reasons. The paper was flagged already in 2014:
Tamar Canello , Kati Frid , Ronen Gabizon , Silvia Lisa , Assaf Friedler , Jackob Moskovitz , María Gasset , Ruth Gabizon Oxidation of Helix-3 methionines precedes the formation of PK resistant PrP PLoS Pathogens (2010) doi: 10.1371/journal.ppat.1000977



In May 2017, the paper was fixed by Gabizon with a Correction:
“The authors would like to correct Fig 4, as changes were made to this figure in preparation for publication that were not indicated in the original figures or figure legends. Specifically, the original mAB 6H4/Sc(RML) Mo panel underwent contrast changes that were not indicated in the original figure or figure legend. The corrected Fig 4 includes a rerun experiment without contrast changes. The authors confirm that these changes do not alter their findings. […]
In addition, Figures 2A, 2B, 2D, 3A, 3B, 5B and 5C underwent a cut and transfer before incubation that was not previously indicated in the figure legends.”
On 11 December 2024, a retraction followed:
“Following the publication and subsequent Correction of this article [1, 2], additional concerns were raised regarding results presented in Figs 2 and 3. Specifically,
- Lanes 1–3 of the Fig 2C 6H4 MO panel appear similar to lanes 1–3 of the Fig 3A mAb IPC1 Mouse Brain panel.
- In the Fig 2C RVC panel, lane 11 appears similar to lanes 4–6 when horizontally stretched.
The authors provided uncropped blots for Figs 2, 3, 4, and 5BC in the Supporting Information files available with the previous Correction of this article [2]. Editorial review concluded that the data provided in [2] do not resolve the additional concerns raised with this article. The underlying mAb IPC1 mouse brain blot for Fig 3A provided with [2] appears to show the same data as the underlying blot provided for the Fig 2C 6H4 panel despite representing different experimental results. Regarding the Fig 2C RVC panel, the underlying data provided for this panel do not fully match the published panel, and underlying data provided for the Fig 2C R1 panel appear to partially match the published RVC panel. The data provided for these panels lend further support for the editorial concerns about the integrity of the data reported for the RVC hamster results and the RVC human E200K results.
The issues were not resolved in follow-up discussions.
Therefore, the PLOS Pathogens Editors retract this article due to concerns about the reliability and integrity of the published results.
RuthG did not agree with the retraction. TC, KF, RonenG, SL, AF, JM, and MG either did not respond directly or could not be reached.
The following additional issues were noted in our editorial assessment:
There appears to be a labelling discrepancy between Fig 3B and its legend, which refer to IPC1, IPC2, RVC (Legend) vs. 6H4, IPC2, RVC (Figure).”
The originally published Fig 4 mAb 6H4 Sc(RML) Mo panel of [1] appears similar to the Fig 6 Anti PrP pAb RTC Mouse RML panel of [3, retracted in 4]. The Fig 4 mAb 6H4 Sc(RML) Mo panel was corrected in [2] but the underlying data for this panel (provided in the S1 File available in [2]) confirm the originally published panel was inappropriately manipulated.
Multiple blots provided with [2] as underlying data for Fig 4 do not appear to match the published panels; these may represent replicate data.”
The PubPeer Stars of Weizmann Institute
Rony Seger, Jacob Hanna, Ilana Kolodkin-Gal, Atan Gross, Sima Lev, Tsvee Lapidot, Moshe Oren, Varda Rotter and others. Let’s celebrate the Weizmann Science!
The reason why PLOS had a second look and retracted that study, were the massive forgeries found in a related PLOS Pathogens paper, which also reused the data from Canello et al 2010, and is now retracted.
It was flagged by Elisabeth Bik on PubPeer in 2015-2016, and was also corrected by Gabizon in 2017.
Yael Friedman-Levi , Zeev Meiner , Tamar Canello , Kati Frid , Gabor G. Kovacs , Herbert Budka , Dana Avrahami , Ruth Gabizon Fatal prion disease in a mouse model of genetic E200K Creutzfeldt-Jakob disease PLoS Pathogens (2011) doi: 10.1371/journal.ppat.1002350
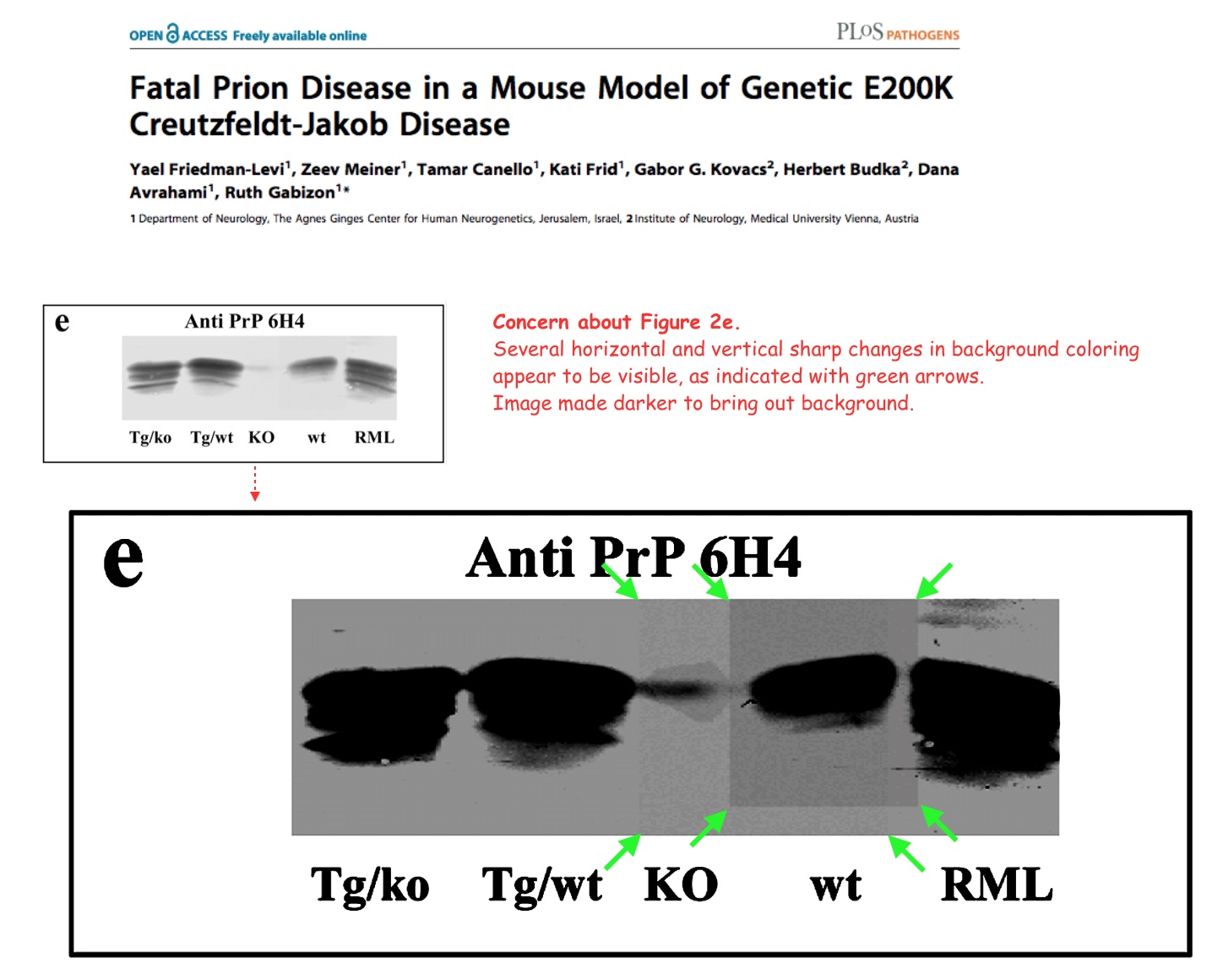
It is only time that tells us if results are good or bad. If they are important, people will repeat them to continue their work. If they are not, they will be forgotten anyway.”


In May 2017, the paper received a Correction for Figure 2E, Figure 5A and 5B, and Figure 2E:
“…The authors confirm that these changes do not alter their findings. The authors have provided raw, uncropped original blots for the corrected figures, Fig 2E, Fig 5A, and Fig 5B. In addition, for full transparency, raw uncropped blots have been provided for other blot figures in the manuscript, including Fig 5C, Fig 6, and Fig 7, as Supporting Information….”
Yet in May 2023, Bik found more and this paper as well as Canello et al 2010 both became untenable:


Maarten van Kampen contributed further analysis of forgeries in that paper, Gabizon retorted on PubPeer by invoking the authority of her Viennese coauthor Herbert Budka, and concluded:
“Since there is no new result in this panel, but just a control which could have been omitted easily from the paper without losing any of the effects regarding the mutant mice, there was no point for “fabrication” of any form, which obviously we did not do.“
On 11 December 2024, the paper was retracted:
“After the Correction was published for this article [1, 2], additional concerns were raised. In following up on the additional issues PLOS also re-examined the results and data reported in the published article and correction. The following issues were noted:
- The wt lane of the originally published Fig 2E [1] appears to be discontinuous with the other lanes. Although the data provided with the Correction notice previously issued on this article [2] appear to support the results, they do not resolve the concerns pertaining to the integrity of the originally published panel. In addition, editorial review of the underlying data provided for Fig 2E [2] suggests that the raw blot provided for this figure appears to be a composite of two images.
- The Correction notice previously issued on this article [2] discloses that panels presented in Fig 5 were prepared using spliced blots and provided original data that appear to support the results presented in panels A-C. However, the explanation and data provided in [2] did not directly address or resolve concerns about the integrity of the Fig 5C total+PK αPrP mAb 6H4 blot in [1]. In addition there appear to be vertical splice lines in Fig 5D [1] which were neither addressed or resolved in [2].
- There appear to be irregularities suggestive of splice lines in the background of Fig 7C, and the lanes in the published Fig 7C panel do not appear to match the underlying data provided in the Correction notice previously issued on this article [2]. Also, the image data provided in [2] suggest that the results reported in lanes 1–2 and lanes 3–8 originated from different blots.
- There appear to be similarities within and between the following panels:
- ○ The Fig 6 Anti Prp pAb RTC WT panel and Anti PrP pAb RVC WT panel appear similar. In addition, for both panels lanes 5–8 appear similar to lanes 9–10 with aspect ratio altered.
- ○ Lanes 3–4 and lanes 9–10 of the Fig 6 Anti Prp pAb RTC TgMHu2ME200K/ko panel appear similar
- ○ The Fig 6 Anti Prp pAb RTC Mouse RML panel of this article [1] and the Fig 4 mAB 6H4 Sc(RML) Mo panel of [3, corrected in 4, retracted in 5] appear similar.
The issues were not resolved in follow-up discussions.
Regarding the overlap between the results in Fig 6 of this article and Fig 4 of [3, corrected in 4, retracted in 5], the corresponding author states that these panels are the same because they represent the same results. Vertical irregularities suggestive of splice lines were observed in both panels, and the corresponding author provided the data underlying the published results. Upon editorial review of the underlying data, PLOS noted that the underlying data provided for the Anti Prp pAb RTC Mouse RML panel confirm this panel was inappropriately manipulated.
In light of the above concerns that raise concerns about the reliability and integrity of the published results, the PLOS Pathogens Editors retract this article.
RG did not agree with the retraction. YFL, ZM, TC, KF, GGK, HB, and DA either did not respond directly or could not be reached.”
Aguzzi and the Lowlifes
The prion researcher Adriano Aguzzi used to describe his Pubpeer critics as “lowlifes”, and himself as a victim of a lynch mob. But after Elisabeth Bik helped him find even more mistakes in his papers, Aguzzi changed his stance.
Speaking of Herbert Budka, professor at Medical University Vienna in Austria. He not only published bad stuff with his fellow prion researcher Adriano Aguzzi (see Nuvolone et al 2017), Budka also has further fake papers with Gabizon in need of retraction:
Yael Friedman-Levi , Romana Hoftberger , Herbert Budka , Tehila Mayer-Sonnenfeld , Oded Abramsky , Haim Ovadia , Ruth Gabizon Targeting of prion-infected lymphoid cells to the central nervous system accelerates prion infection Journal of Neuroinflammation (2012) doi: 10.1186/1742-2094-9-58
Fig 1a, by Actinopolyspora biskrensis

This was reported on PubPeer already in 2014:
Yael Friedman-Levi , Haim Ovadia , Romana Hoftberger , Ofira Einstein , Oded Abramsky , Herbert Budka , Ruth Gabizon Fatal neurological disease in scrapie-infected mice induced for experimental autoimmune encephalomyelitis Journal of Virology (2007) doi: 10.1128/jvi.00780-07
“Figure 1a. The 10exp-6 lane appears to consist of two identical-looking areas.”

And Gabizon? Having been caught out a decade ago, do you think she became more careful? Nope.
Fake data and real pomegranate juice in Nobelist Louis Ignarro’s papers
Louis J. Ignarro knew how to monetize his 1998 Nobel Prize for discovery of nitric oxide as molecular cell signalling agent. He made many millions selling dietary supplement for Herbalife and pomegranate juice for POM Wonderful Company. Some of that found its way (without proper conflict of interest declaration) into Ignarro’s peer reviewed papers. Those,…
In fact, she is pushing “Nano formulation of Pomegranate seed oil [Nano-PSO (Granagard TM)“]” by the own company Granalix, here as a cure for brain trauma:
Doaa Qubty , Kati Frid , Meirav Har-Even , Vardit Rubovitch , Ruth Gabizon , Chaim G Pick Nano-PSO Administration Attenuates Cognitive and Neuronal Deficits Resulting from Traumatic Brain Injury Molecules (2022) doi: 10.3390/molecules27092725
Dysdera arabisenen: “Fig 6 Nano-PSO treated control and Nano-PSO treated post-TBI seem to have the same images.”

To add insult to traumatic brain injury:
“The authors declare no conflict of interest.“

Concerns regarding the veracity
Scientific Reports removes artwork, something from Saudi Arabia about oil drilling by some Salaheldin Elkatatny, a professor King Fahd University of Petroleum & Minerals (h-index 50):
Salem Basfar , Salaheldin Elkatatny Micronized calcium carbonate to enhance water-based drilling fluid properties Scientific Reports (2023) doi: 10.1038/s41598-023-45776-y

On 10 December 2024, this cack-handed cartoon was retracted :
“The Editors have retracted this Article.
After publication, concerns were raised regarding the veracity of the following data presented in this work,
- the X-ray diffraction (XRD) pattern labeled Conventional CaCO3 shown in Figure 4;
- the statistical analysis of the effect of calcium carbonate on the density of the water-based drilling fluid, shown in Figure 5.
The Authors provided raw data on request from the Editors. However, the Editors found that the original XRD pattern is different from that presented in the Article. The Editors therefore no longer have confidence in the research presented in this work.
None of the Authors have responded to correspondence from the Editors about this retraction.”
Donate!
If you are interested to support my work, you can leave here a small tip of $5. Or several of small tips, just increase the amount as you like (2x=€10; 5x=€25). Your generous patronage of my journalism will be most appreciated!
€5.00















What? No other comments? I think of Sinclair as something of a sacrificial lamb. With enough bad press, the other members of the Academy for Health and Lifespan Research (AHLR) thought it prudent to distance themselves from him (amputate, perhaps is a good metaphor). If anything, the AHLR is a society of mutual protection, reinforcing the interests of other members by lending legitimacy to their research and ensuring access to research and investment dollars. Competition between ideas based on rigorous fact checking was once integral to scientific integrity. Now these formidable scientists of the AHLR, many who were once critical (and rightly so) of the ideas espoused by other members, give their hearty assent.
LikeLike
The concerns raised about figures like David Sinclair and organizations like the Academy for Health and Lifespan Research (AHLR) resonate with broader critiques of the interplay between science, investment, and public perception. This dynamic often involves leveraging the credibility of scientific research to attract funding, sometimes veering into questionable or exaggerated claims. The “pump and dump” cycle is a troubling phenomenon that can undermine trust in science and lead to financial and ethical missteps.
In the case of Cassava Sciences, allegations have surfaced accusing the company of manipulating research data related to its Alzheimer’s drug, Simufilam, to inflate its stock price. While the company denies wrongdoing, the controversy highlights how scientific claims can be weaponized for financial gain, potentially at the expense of patients, investors, and the integrity of the scientific process. It serves as a stark reminder of the importance of transparency, independent verification, and rigorous peer review in science.
David Sinclair’s role as a prominent advocate for anti-aging research has also attracted scrutiny, with critics arguing that some claims are overstated or not adequately supported by robust evidence. If the AHLR operates as a “mutual protection society,” as you suggest, it raises further concerns about whether the competitive rigor that traditionally fuels scientific advancement is being compromised by a culture of endorsement and self-interest.
The convergence of scientific research, market forces, and media amplification creates fertile ground for the propagation of both genuine breakthroughs and unsubstantiated hype. Restoring trust in this system requires an uncompromising commitment to fact-checking, ethical standards, and open discourse, ensuring that scientific progress is driven by data rather than dollars.
Happy winter festivities!
LikeLike
I hope one day someone will take me by the hand and point me towards a genuine breakthrough in anti-aging. Or if not a breakthrough, to something which is not complete bullshit.
LikeLike
Wrt human longevity breakthroughs, it seems atm there’s nothing better than ‘not sitting on your ass’ all the time, ‘put the fork down’ every now and then, don’t be insufficent in essential nutrients and don’t overwhelm your detox system.
Being a rodent in an anti-aging lab might offer profound longevity advantages. However, there might be a few trade-offs.
Regarding anti-aging, some traditional methods, such as hormone replacement therapy (including a small amount of HGH), can work wonders for improving appearance, vitality, and performance relative to your age group.
Doesn’t make you live longer. But hey YOLO!
Disclaimer: The above comment represents a personal, unscientific opinion from a layperson and should not be taken as medical advice. Always consult a qualified healthcare professional for guidance on health and longevity.
LikeLike