Frontiers is really the best scholarly publisher in the world. Honestly, I mean it. My eyes are open now, I understood that science was never about actual science, but about publishing papers to advance your personal career, business and agenda, at all costs. And Frontiers is really the best service provider out there, scientists, businesspeople and loonies have understood long ago that their €3k publication costs will be well invested with Frontiers. With scientific publishing, it’s not what kind of science is inside (if any), it is how it’s wrapped and marketed, and Frontiers is truly the best there. If Frontiers was a fashion brand, we would all run around stark bollock naked with feathers stuck up our bums.
Well, it’s not just fun and games. Sure, Frontiers publishes masses of silly stuff, their outlandish autism theories are classic, their paranormal activities on clairvoyance and life after death are comedy gold, and what better way to go into the pandemic if not the hilariously unhinged yet rigorously peer-reviewed antivaxxery by Frontiers?
So of course it is not surprising that Frontiers apparently seeks to join the COVID-19 carnage with quack medicines. Pity that the chloroquine guru Didier Raoult already has (courtesy of Elsevier) his own set of journals, with much faster peer review and higher impact factors, and doesn’t need to waste time and money on Frontiers. Having failed with Pierre Kory and his ivermectin, the Swiss Open Access publisher now serves you some of the most dangerous quacks around: those of the proxalutamide team.

The story of the hair-loss drug proxalutamide, made by a Chinese pharma company, marketed and patented by a Californian mailbox startup, repurposed by chloroquine-battle-steeled Brazilian doctors as a COVID-19 miracle cure, and touted by Brazil’s fascist president Jair Bolsonaro, is told in this article.
So what follows is a kind of extensive update, some of it already reported in earlier Schneider Shorts.
Frontiers in Homicidal Quackery
Now, what happens to fraudulent and unethical clinical trials where investigators are friends with a fascist dictator and have been poisoning their patients with various quack COVID-19 therapies?
Well, they publish their breakthrough in Frontiers of course, an investment of measly €3k, and then these crooked Brazilian doctors and their Chinese pharma partners cash in big time.
John McCoy , Andy Goren , Flávio Adsuara Cadegiani , Sergio Vaño-Galván , Maja Kovacevic , Mirna Situm , Jerry Shapiro , Rodney Sinclair , Antonella Tosti , Andrija Stanimirovic , Daniel Fonseca , Edinete Dorner , Dirce Costa Onety , Ricardo Ariel Zimerman , Carlos Gustavo Wambier Proxalutamide Reduces the Rate of Hospitalization for COVID-19 Male Outpatients: A Randomized Double-Blinded Placebo-Controlled Trial Frontiers in Medicine (2021) doi: 10.3389/fmed.2021.668698
The study declared:
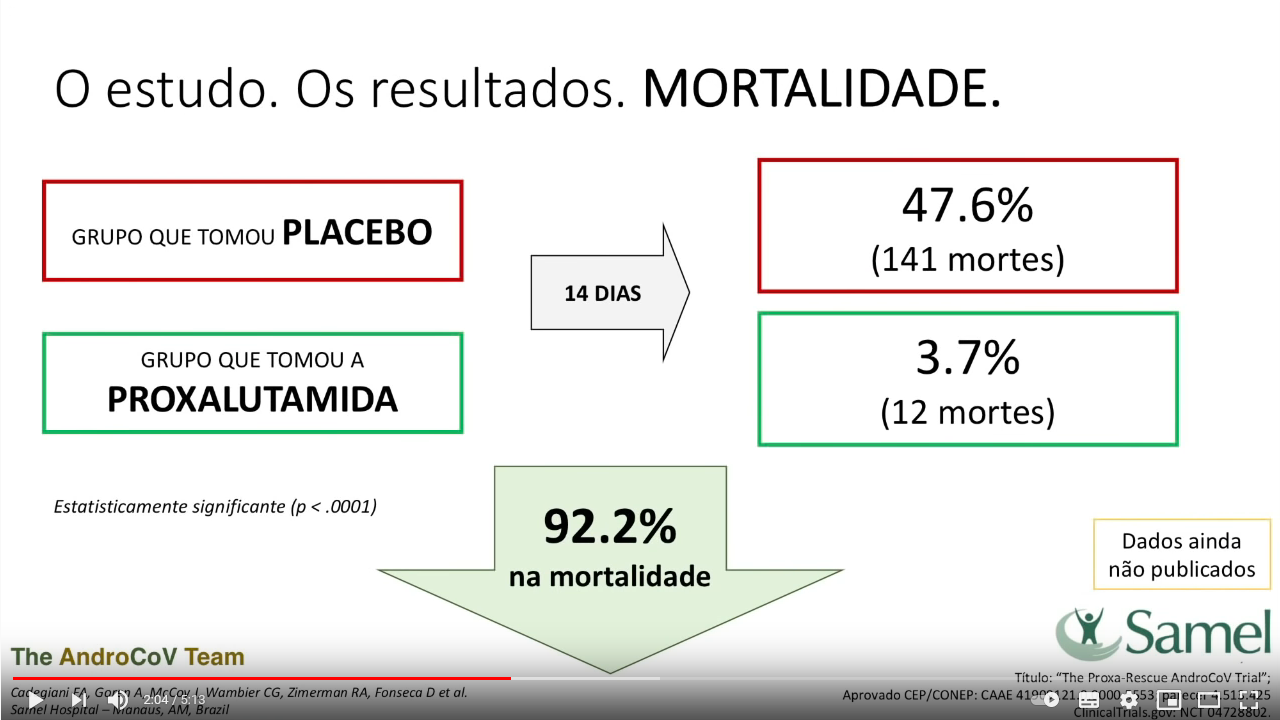
“A total of 268 men were randomized in a 1:1 ratio. 134 patients receiving proxalutamide and 134 receiving placebo were included in the intention-to-treat analysis. [….] Here we demonstrate the hospitalization rate in proxalutamide treated men was reduced by 91% compared to usual care.”
That same toxic trash was already debunked as fraudulent in its preprint version on PubPeer and by Smut Clyde on my site. Thing is, before proxalutamide, the doctors used another anti-androgen, dutasteride, this goes unmentioned in the methods section of the Frontiers paper. And their control arm was the anti-parasitics ivermectin and nitazoxanide, plus the antibiotic azithromycin, plus many other drugs, probably mixed in a bucket, which would explain the initially announced almost 50% mortality in the control arm.
Here is Smut Clyde trying to untangle things (I inserted the hyperlinks):
“I have spent probably too much time today trying to understand what happened with the Dutasteride clinical trial. It evolved in many ways. Notably, between 23 June and 25 July 2020 it acquired a third group of 120 patients, who would receive Proxalutamide (in addition to Ivermectin and Azithromycin). Presumably this was on the insistence of Kintor Corporation, manufacturers of Proxalutamide, who were searching for an application for their drug (in addition to “hair loss” and “androgen-blocker-resistant prostate cancer”), and stepped in to fund the study – as noted in their press release. The job of Advanced Biology was now to find that application.
Between July 28 and 10 December, the useless Ivermectin treatment was dropped and replaced with Nitazoxanide. I have no idea why.
Between 10 and 29 December, as the end of the trial approached, the researchers discovered that patients had not been systematically treated with Ivermectin, Nitazoxanide OR Azithromycin, and the ‘background treatment’ aspect was replaced with “Standard of care as determined by the PI”. Patients could be receiving ANYTHING and still be eligible.
Meanwhile, all the outcomes were dropped except “Percentage of subjects hospitalized due to COVID-19”. All other measures? No longer relevant. Suspicious minds might leap to the conclusion that those other measures showed no difference.
‘Presenting “Gabrin sign” i.e., androgenetic alopecia’ was dropped as an inclusion criterion. “Male pattern boldness” was the whole rationale for the study, but it just vanished.
Oh yes, and the Dutasteride group just disappeared. Now only two groups in the study, comparing Proxalutamide with placebo. What happened to all the patients previously treated with dutasteride?
The result is to make nonsense of various preprints [Cadegiani et al medRxiv 2020, Cadegiani et al ResearchSquare 2020] comparing dutasteride and placebo, which refer NCT04446429 for the details and ethics approval. These cite 130 subjects (64 in the D group and 66 placebos), or a subset of 87 who were studied in more detail (43 Ds, 44 placebos).
But wait, there’s more! A second Applied Biology clinical trial exists for dutasteride: NCT04729491. No ethics approval is mentioned. The study finished in 15 September, and was retrospectively registered on 28 January. Apparently this is possible.“
No results are presented in the CT entry, but this is the trial cited in other dutasteride papers and preprints. Apparently it is also possible, at the end of a clinical trial, to extract inconvenient cases and reassign them to a second, newly-spawned trial. I don’t make the rules.”
The Frontiers paper divulges some information about the standard care:
“Usual care consisted of various combinations of medications and was individualized to each patient’s needs. Azithromycin 500 mg per day for 5 days and nitazoxanide 500 mg twice a day for 6 days were offered to all new study patients. The following medications were prescribed as needed: dipyrone, paracetamol, ondansetron, dexamethasone, rivaroxaban, and enoxaparin. In cases of hospitalization, additional drugs were prescribed according to the clinical judgement of the hospital staff. Treatment compliance with study drugs was not monitored during the trial. Usual care medications prescribed by doctors outside of the trial were not documented.”
Well, here it is, the usual care medication as prescribed by Dr Cadegiani to his hapless COVID-19 patients, as a combo: malaria drug hydroxychloroquine, anti-parasitics ivermectin and nitazoxanide, antibiotic azithromycin, anti-depressant fluvoxamine, anti-inflammatory drugs colchicine and dexamethasone, anti-androgens bicalutamide, spironolactone and dutasteride, heartburn drug dexlansoprazole, anti-coagulant apixaban, Vitamin D and Vitamin C, plus maybe who knows what other drugs.

The mass-dying in the control arm subsided somewhat in the Frontiers edition of the scam, but the significance remains the same: over 90% lives saved! Now, it is close to improbably that Frontiers had no clue of the constant fraud accusations against the proxalutamide team. Even if they missed my reporting or the Pubpeer discussion: a Brazilian article from 9 April 2021, original in Portuguese, titled:
“Bolsonaro’s ‘new chloroquine’ study has evidence of fraud and serious flaws“
I was very proud to see a reader of my site quoted:
“For this reason, researchers such as Jose Galluci Neto, an assistant physician who works at the Institute of Psychiatry of the USP School of Medicine, say that the study period is not feasible. “It is impossible”, summarizes Gallucci. “It is not a deadline at all.“”
Later on, Brazilian journalists of Globo wrote in June 2021:
“….,the information revealed aroused in specialists suspicions of fraud and serious flaws – such as the death of a high number of volunteers, which should have led to the immediate suspension of the research. In its investigation, Conep [National Research Ethics Commission] found that the case is even worse. All premises of the protocol submitted to the council by the proxalutamide researchers were not complied with. In practice, they did everything differently than they promised the ethics committee.
To begin, the researchers, led by the physician Flávio Cadegiani, registered the study as if it were going to be carried out in hospitals in Brasília, but they did it in Manaus and other cities in the Amazon.” They took the study at their own risk to the Amazon and distributed the medicine in a series of hospitals in the interior of the state without any approval”, explained a Conep advisor.
In addition, the protocol planned to test proxalutamide in less than 300 patients with moderate Covid, but the authors changed the direction of the research without informing Conep and applied the drug to 615 severely ill patients.
As if it were not enough, only when they submitted the final data to the commission, in May, the authors reported the occurrence of “more than 200 deaths”. Thus, they failed a Conep standard, dated May 2020, which establishes that the deaths of volunteers must be reported to the commission within 24 hours.
The death rate is also much higher than the 141 deaths that the researchers themselves reported at the press conference. At the time, they said most of the deaths would have occurred among volunteers who received the placebo – which would prove 92% effectiveness.”
Basically, Cadegiani’s standard care therapy was even more lethal than originally thought.
The proxalutamide study is toxic in many respects. But Frontiers was more than happy to publish it. In this regard, BBC News Brazil offers some background:
“The authors published their findings on Monday (19/7) in a study (already peer-reviewed) in the journal Frontiers in Medicine, after being rejected by prestigious scientific publications such as The New England Journal of Medicine and The Lancet.”
For Better Science is referenced in that BBC article. At the end we learn that Bolsonaro and his government are still pushing proxalutamide as COVID-19 medicine, regardless of fraud and patient abuse accusations.
Homicidal Maniacs
Time to briefly introduce the team behind the Frontiers paper.
- Andy Goren is founder and apparently sole owner of Californian start-up Applied Biology, which postal address is just a mailbox and which before the pandemic focussed on scamming with hair loss cures. He pretends to be merely an employee. The company has a certain Torello Lotti on board, an Italian quack so dangerously insane he was even arrested and put on trial. John McCoy is Vice-President of Applied Biology, another Applied Biology’s employee is Maja Kovacevic, she declares no conflict of interest (COI).
- Flavio Cadegiani: self-proclaimed genius and scientific director of Applied Biology, see above his homicidal COVID-19 standard care regimen. Also co-inventor of the homicidal Brazilian COVID-19 app TrateCov, more on that below.
- Ricardo Zimerman, Brazilian military clinician and homicidal maniac who drove in Porto Alegre region the mass prescriptions for hydroxychloroquine, ivermectin, vitamin D and zinc (made standard therapy under his pressure) while opposing all COVID-19 restrictions. The resulting COVID-19 carnage in Brazil, especially in Manaus and the Amazonas state, is also his direct responsibility. Zimmermann has a staggering amount of blood on his hands.
- Carlos Wambier is a Brazilian-born dermatologist at Brown University in USA and also Applied Biology board member, yet he declares no COI.
- Jerry Shapiro and Rodney Sinclair are two other Applied Biology board members, both hair-loss dermatologists with his own private clinics, one in North America and the other in Australia.
The Brazilian clinical scientist Jose Gallucci-Neto commented on my site what else Cadegiani has been up to:
“Cadeggiani is also probably behind (1) the AndroCov (latterly renamed as TrateCov) killer app (using RedCap) released officially by the Brazilian Ministry of Health to “prescribe” the infamous “COVID kit” (HCQ, AZT, Ivermectine, Zn, etc., etc.) to the population. According to the Federal Council of Medicine (2) the app violated many ethical issues. The app could also imply in a crime of responsibility (2) to president ‘BolsoNero’, according to the analysis below. The TrateCoV app indicated chloroquine and other ineffective treatments to almost all patients (including toddlers and babies!!). The word chloroquine appears 86 times in the app code and ivermectin 113 times.
Galucci-Neto also provided a translation of the relevant article from January 2021, excerpt:
“ After analysis carried out by technical and legal advisors and advisers on the TrateCov application, recently launched to assist teams in collecting symptoms and signs from patients possibly infected with covid-19, the Federal Council of Medicine (CFM) alerted the Ministry of Health about the following inconsistencies in the tool:
• It does not adequately preserve the confidentiality of information;
• Allows it to be filled out by non-medical professionals;
• Ensures scientific validation for drugs that do not have this international recognition;
• Induces self-medication and interferes with doctors’ autonomy;
• It does not make clear, at any time, the purpose of using the data filled in by the attending physicians.”
“The Ministry of Health withdrew the TrateCov application platform from the air on Thursday (1/21). The tool is intended for the guidance of health professionals and was available for a week. The tool recommended the early treatment of people with Covid-19 symptoms with drugs without scientific evidence – such as chloroquine and ivermectin.
After removing the app, the Ministry of Health stated, in a note, that the application had been “hacked and activated improperly” and that the platform was launched as a pilot project and was not officially functioning as a simulator.”
But of course the nation-wide quackery of Cadegiani and Zimerman would not be possible without the support from the very top, from Brazil’s fascist leader, Jair Bolsonaro. Reuters reports about the Brazilian dark eminence who made the connection: Helio Angotti, described as “a little-known Health Ministry official” appointed by Bolsonaro himself. Angotti and the president are both followers of “the esoteric Brazilian philosopher Olavo de Carvalho, who promotes false conspiracy theories including a claim that Pepsi used the cells of aborted fetuses as sweetener“.
According to Reuters, Angotti is under federal investigation in Amazonas state for administrative misconduct “for pushing health workers there to prescribe hydroxychloroquine“. His aim seems to be to cause maximal chaos and carnage by spreading covidiot denialism against vaccines, lockdowns, face masks and in support of quack cures, and Cadegiani and Zimerman are exactly the right thugs for this bloody job. The latter was one of the three consultants Angotti hired to prove Bolsonaro’s chloroquine vision right.
Zimerman was sent by Angotti to manage the mass murder in Manaus by forcing hospitals to use hydroxychloroquine during the oxygen shortage, while Cadegiani was tasked with poisoning Brazilians, even children, via the chloroquine-peddling TrateCov app, as Reuters reported:
“The Health Ministry sent at least 120,000 hydroxychloroquine pills to Amazonas state and flew 12 medical professionals to the city of Manaus to push healthcare workers to use anti-malarials. Zimerman, De Souza and Costa were in the group, according to a Feb. 23 statement sent by one of Angotti’s colleagues to federal prosecutors in Amazonas, responding to their inquiries about the ministry’s handling of the Manaus crisis. Angotti’s SCTIE financed their trips, the statement says.
The ministry also deployed a short-lived phone app that purported to help medical professionals diagnose COVID-19 with a symptoms questionnaire – then instructed them to prescribe anti-malarials like hydroxychloroquine. The app was based on a diagnostic tool that Angotti’s consultants helped to develop.”
But what do I know, Frontiers are the real experts in research integrity. If they say all these accusations against Cadegiani, Zimerman et al are lies…
Rotten System
Frontiers is merely a symptom of an utterly rotten scholarly publishing system, which is beyond repair. It was Elsevier and the allegedly learned International Society of Antimicrobial Chemotherapy (ISAC) which allowed Raoult’s illegal chloroquine quackery.
Another learned society decided to push proxalutamide.
This is the recent COVID-19 position paper from the European Society of Endocrinology (ESE) to outline “the prevention and management of the disease“:
Puig-Domingo M, Marazuela M, Yildiz BO, Giustina A COVID-19 and endocrine and metabolic diseases. An updated statement from the European Society of Endocrinology. Endocrine (2021) DOI: 10.1007/s12020-021-02734-w
This is the relevant quote from it:

“Data from the dermatologic literature suggest that bald men are more prone to COVID-19 [128] as well as that androgenetic alopecia is frequent also in women with COVID-19 [129]. However, one of the more impactful observations in this field came from patients with prostate cancer. In fact, those patients under androgen deprivation therapy [130] demonstrated in some studies to be less vulnerable to SARS-CoV-2 infection, as compared to patients who were not androgen deprived [131]. Most intriguingly, recent double-blind placebo-controlled trials showed that selective androgen receptor modulators accelerates viral clearance and reduce time to clinical remission in male patients without prostate cancer hospitalized with mild to moderate COVID-19 as well as a novel androgen receptor inhibitor did show similar positive effects in both males and females with COVID-19 [132, 133].“
The references 128-133 are to those Cadegiani, Zimerman, Wambier et al studies. I informed the European Society of Endocrinology leadership and the paper’s corresponding author about the suspected fraud and patient abuse: NONE of them replied.
And then of course, the proxalutamide scam was not Frontiers’ first attempt to sabotage the pandemic relief efforts. Before, it was ivermectin.
To be fair, the following one-paragraph paper in Frontiers by the US quack Pierre Kory, president of the so-called Frontline COVID-19 Critical Care Alliance (FLCCC), was rather short-lived. It saw the light of the day on 13.01.2021, and went extinct on 1.03.2021:
Pierre Kory, G U. Meduri, Jose Iglesias, Joseph Varon, Keith Berkowitz, Howard Kornfeld, Eivind Vinjevoll, Scott Mitchell, Fred Wagshul and Paul E. Marik Review of the Emerging Evidence Demonstrating the Efficacy of Ivermectin in the Prophylaxis and Treatment of COVID-19 Front. Pharmacol. doi: 10.3389/fphar.2021.643369
There were namely big protests on social media, so the Kory paper was ERASED by Frontiers, not just retracted. It is gone completely. The only trace of its past existence is a silly Frontiers editorial, posted only after journalists started asking. The whole idea of permanency attached to a DOI does not apply to troll publishers liek Frontiers, so here is a WayBack Machine archived record. And here is a screenshot:

The piece of garbage was the re-published in American Journal of Therapeutics issued by Wolter-Kluwer because this is how scientific publishing works. It was then reported by many news outlets (eg CNBC or Times of India) celebrating that Kory’s ivermectin bunk passed peer review, without mentioning the Frontiers cock-up.
Money Shower
The investment of €3k into that Frontiers paper paid out hundreds of thousands fold for the proxalutamide doctors and businessmen. The Chinese company Kintor Pharmaceutical which sells proxalutamide and pays Applied Biology, has received an COVID-19 Emergency Use Authorization (EUA) for proxalutamide in Paraguay, as per recent press release:
“The first hospital to use proxalutamide under the EUA, Hospital Barrio Obrero, part of Paraguay’s MSPBS network, has reported promising initial results. Of the first 25 COVID-19 inpatients treated with Proxaluatmide, only 1 patient (4%) died. Such mortality rate was lower than the usual mortality rate of COVID-19 inpatients in Paraguay. In addition, proxalutamide has demonstrated potential benefits in 7 of the patients who initially required high flow oxygen”
Paraguay is just the beginning, thank you Frontiers. Here is another press release:
“Kintor Pharmaceutical Limited (HKEX:9939) today announced that it has entered into a licensing agreement with Shanghai Fosun Pharmaceutical Development Ltd. (“Fosun Pharma Development”), on the commercialisation of proxalutamide for the treatment of COVID-19 indication in India and 28 African countries (the”Collaboration Regions”). Kintor and Fosun Pharma Development will collaborate on the emergency use authorization applications, promotion, and sales of proxalutamide for the treatment of COVID-19 indication.”

Get For Better Science delivered to your inbox.
Make a one-time donation
Make a monthly donation
Choose an amount
Or enter a custom amount
Your contribution is appreciated.
Your contribution is appreciated.
DonateDonate monthly

Frontiers are also happy to publish advertisements for Ayurvedic garbage.
https://www.frontiersin.org/articles/10.3389/fphar.2021.623795/full
LikeLike
“Ayurvedic medicines, especially Ashwagandha (Withania somnifera (L.) Dunal, WS), may be beneficial in the management of COVID-19. WS is a widely prescribed Ayurvedic botanical known as an immunomodulatory, antiviral, anti-inflammatory, and adaptogenic agent. The chemical profile and pharmacological activities of WS have been extensively reported. Several clinical studies have reported its safety for use in humans. This review presents a research synthesis of in silico, in vitro, in vivo, and clinical studies on Withania somnifera (L.) Dunal (WS) and discusses its potential for prophylaxis and management of COVID-19.”
Edited by
Jia-bo Wang
School of Traditional Chinese Medicine, Capital Medical University, China
I’m afraid Mr Smut, this is rigorously peer-reviewed science, but sure, you are welcome to disprove it the scientific way. Which Ayurvedic herb would you rather propose against COVID-19 then?
LikeLike
That’s Dr Smut!
LikeLiked by 2 people
Well, I guess it’s pretty evident judging by the lack of comments to your article that you’re not being taken very seriously.
LikeLike
It’s just published and you already commented. QED.
LikeLike
Reply from Frontiers in Medicine Editor-in-Chief Michel Goldman:
“First of all, let me tell you that I have not been involved by whatever means in the publication of this paper.
I will now ask the Editorial manager of Frontiers in Medicine to review the editorial process which led to the acceptance of this paper for publication in our journal.“
LikeLike
I tried to warn people.
LikeLiked by 3 people
Pingback: Variante Z – ocasapiens
LOL
LikeLike
From past Schneider Shorts:
Querdenker at Frontiers
Of course, MDPI has been merely emulating Frontiers, while going further down. In 2020, an anti-lockdown paper from France proclaimed:
Quentin De Larochelambert, Andy Marc, Juliana Antero, Eric Le Bourg, Jean-François Toussaint Covid-19 Mortality: A Matter of Vulnerability Among Nations Facing Limited Margins of Adaptation Frontiers in Public Health (2020) doi: 10.3389/fpubh.2020.604339
The argument being that lockdowns cause even more disease by preventing people from sports exercise (presumably, in cinemas, pubs, restaurants, clubs and conferences).
The lead author Jean-François Toussaint is professor in Paris and a friend of Didier Raoult and Christian Perronne, the three gentlemen joined Nobelist Luc Montagnier to spread covidiocies in a January 2021 conference.
Meanwhile, Frontiers started to worry that their contribution to pandemic efforts evoked too much ridicule, and worse, angry protests. So Frontiers first erased an ivermectin quackery paper by Pierre Kory (read here), and then cancelled the entire collection on repurposed drugs (here backup) it was part of. Five editors, led by the US consultant Robert Malone, resigned in protest, even though Malone’s own contribution, peddling the heart-burn drug famotidine, remained online (Malone et al 2021). But, The Scientist writes:
Malone et al made an ultimatum to Frontiers demanding immediate publication, but it was a bad time to push embarrassing pandemic quackeries, peer reviewed or not. Hence the issue cancellation and the five resignations.
Poor Frontiers. The serious scientists left, and now the unserious ones are leaving also.
LikeLike
How do you dare telling such lies? The “conference” with Toussaint, Raoult and other guys did not happen, as the organisers “forgot” to tell to speakers they were expected to give a talk. This is known from the French press for months, and it is the duty of a journalist to check infos, and not to provide fake news. In addition, the paper by de Larochelambert et al. (2020) is not an “anti-lockdown” paper, but a study of Covid-19 mortality up to end of August 2020 in most of countries, restricting measures being only one of the many studied variables. You say “serious scientists”? Not you, for sure.
LikeLike
How do you dare, too. The most inept defense of Didou so far!
LikeLike
Proxalutamide save you from COVID, protect your testicle and increase your libido, according to one of the authors. Where can I buy it?
“Proxalutamide is protective to the testicles. Besides testosterone, all major inflammatory and thrombotic markers improve with te drug. My research is available on @ResearchGate”
https://bityli.com/L3KCA
LikeLike
https://www.researchgate.net/publication/353430192_Proxalutamide_Improves_Inflammatory_Immunologic_and_Thrombogenic_Markers_in_Mild-to-Moderate_COVID-19_Males_and_Females_an_Exploratory_Analysis_of_a_Randomized_Double-Blinded_Placebo-Controlled_Trial_
LikeLike
It seems now that even Kintor is “uncomfortable” with the results from the Cadegiani and Zimerman teams. The company applied to ANIVSA for a phase 3 proxalutamide RCT without using/sending the data from the Manaus study that claimed 92% death reduction in the proxalutamide group. Is Kinto trying to dissociate its image from these scientists? Probably.
“The Chinese Kintor Pharmaceutical, the maker of proxalutamide, confirmed not having sent the results of Amazonas to Anvisa. The company claims that state data were excluded because they referred to hospitalized volunteers, while the trial now registered at Anvisa will follow outpatients.
In the note with the answers about the case, the company emphasizes that the current study is “independent,” unrelated to the trial carried out in Manaus.
For researchers heard by the report, however, Kintor’s justification is not consistent with the practice of other clinical studies. “For what reason are you going to drop a study that saves 92% of critically ill patients? If this finding is true, the data could have been leveraged. Disposal raises even more suspicions”, says José Gallucci-Neto, researcher and assistant physician at the Psychiatry Institute of the USP Medical School.”
It seems that Kintor itself suspects that the researchers acted without the methodological rigor.”
In today’s report from “O Globo”
https://blogs.oglobo.globo.com/malu-gaspar/post/fabricante-da-proxalutamida-exclui-estudo-com-indicios-de-fraudes-de-dossie-enviado-anvisa.html
LikeLiked by 1 person
Now we have a bizarre note from Brazilian regulatory agency (Anvisa):
ANVISA clarifies that so far two clinical studies have been authorized to be carried out in Brazil with proxalutamide (GT-0918) for the treatment of Covid-19. The studies, sponsored by the company Suzhou Kintor Pharmaceuticals, based in China, are:
MULTI-REGIONAL PHASE 3, RANDOMIZED, DOUBLE-BLIND, PLACEBO-CONTROLLED STUDY TO ASSESS THE EFFECTIVENESS AND SAFETY OF GT0918 IN THE TREATMENT OF MALE PATIENTS WITH COVID-19 LIGHT TO MODERATE. VERSION 2.0, 21MAR2021. PROTOCOL GT0918-MT-3001. Authorized on June 10, 2021.
PHASE 3, RANDOMIZED, DOUBLE-BLIND, PLACEBO-CONTROLLED STUDY TO ASSESS THE EFFECTIVENESS AND SAFETY OF PROXALUTAMIDE (GT0918) IN MALE AMBULATORY PARTICIPANTS WITH LIGHT TO MODERATE COVID-19. VERSION 1.0, MARCH 18, 2021. PROTOCOL GT0918-US-3001. Authorized on July 19, 2021.
For approval of these studies, efficacy and safety data for prostate and breast cancer indications conducted in other countries were presented.
In the documentation submitted to Anvisa, a study conducted in Brazil for Covid-19 (NCT NCT044464290) by the initiative of independent researchers was cited, whose data were not made available. The Agency was also told that a study in critically ill patients was ongoing (NCT04738802), but no report or results were presented.
These studies were not considered for Anvisa’s approval for the clinical research to be carried out and also cannot be submitted for the purpose of eventual drug registration, as these are studies whose data are not subject to regulatory assessment.
Considering the data presented and the design of studies approved to assess the safety and efficacy of proxalutamide, the benefit x risk profile remains favorable for the continuation of studies, until new data are presented.
https://www.gov.br/anvisa/pt-br/assuntos/noticias-anvisa/2021/esclarecimentos-sobre-estudos-com-proxalutamida-aprovados-pela-anvisa
LikeLiked by 1 person
Who would have thought, the Cadegiani et al clinical trial was fraudulent. The bigger scandal is that the patients and the treatments were real.
Hattip Smut Clyde and Jose Galucci-Neto
LikeLiked by 1 person
Brazilian “ProxaTuskegee” scandal!
It seems we have another shameful chapter of proxalutamide fraud. According to a recent report from @PedroNakamura, Caddegiani and Zimerman held an obscure RCT named “ProxaSouth” testing proxalumatide in a military hospital in Porto Alegre without ethical approval from Brazilian authorities (CONEP and ANVISA). When? March/21!
https://www.matinaljornalismo.com.br/matinal/reportagem-matinal/proxalutamida-hospital-militar-covid-porto-alegre/
According to Caddegiani, who needs ethical bureaucracies for an RCT with an experimental drug when you know you are saving lives?
“We decided that obstacles and inadequate bureaucracies cannot further delay the dissemination and publication of results of great importance for global public health, in the midst of a pandemic. Certain types of deficiency in institutions cannot stop the advance of research that is strictly ethical and even mandatory from a moral point of view, in view of the results”, they wrote in posts on social networks, as a justification for launching Proxa South. Asked if the release had something to do with the contact for the report, Cadegiani’s staff replied, by phone, that the Matinal “only reminded the doctor to disclose the study’s data.”
The blindness of the study was compromised as usual -see the third picture embedded in the text
“Proxalutamine pills delivered by doctors from the Hospital da Brigada Militar to a military police officer hospitalized by Covid-19 contain the batch number and drug code – which proves that blindness has been broken”.
An anonymous patient (Eva) told the reporter that she was told to continue the use proxalumatide after hospital discharge without any follow-up from the researchers.
“After just over a week in the hospital, Eva was discharged. She returned home with a prescription to take 300 mg of the experimental drug daily until he completed two weeks of continuous use. Eva considered the dosage high but took it anyway. “They had only explained to me the name of the drug and that I was supposed to take three pills,” she said. As proxalutamide is a new drug, it is not known how different it is from the antiandrogens available on the market.”
“At home, Eva spent two weeks with severe weakness in her legs and mental confusion, supposedly after-effects of the virus. After two weeks, she recovered and even wondered if this evolution could have anything to do with the medicine. The doctors at the Hospital da Brigada Militar had promised to monitor the patient’s health after the tests – but no one came to see her. “They didn’t contact me later. I found this strange. If they did the follow-up research, they should be interested in knowing if the patient got worse, got better, died, but they didn’t look for me anymore. I even found the lack of interest in the results strange, as I improved, which would be great”, she said.”
“However, months after the experiment, Eva reported having “severe” hair loss, without being sure why. “My dermatologist said it was likely to be a consequence of Covid,” adds the patient. One of the functions of medical follow-up would be precisely to assess the long-term safety of the drug being tested.”
LikeLiked by 1 person
In this regard:
LikeLiked by 1 person
Pingback: Médico negligenciou riscos de malformação fetal por proxalutamida em pacientes com covid - Matinal Jornalismo
Pingback: Una lobby, due nomi – ocasapiens
New York Times reports from Brazil:
As El Pais Brazil writes:
You can read about Cadegiani’s fraud and horrendous ethics breaches here and here. Among other things, he is accused of experimenting on people without ethics approval and of causing the deaths of 200 patients in the control group of his COVID-19 trials with the anti-androgen proxalutamide, which is marketed by the US company Cadegiani works for, Applied Biology. The patients in the control arm apparently were poisoned with a bizarre mix of all possible drugs Cadegiani and his partners declared to be “standard care”.
In fact, Cadegiani refuses to tell the National Research Ethics Commission (Conep) what he gave his control group patients which then caused their deaths, a sthe National Health Council press release hints:
The El Pais article also discusses a certain case report Cadegiani et al published in a BMJ journal:
This was the case report about this “compassionate use” case which however never had an ethics approval from the Brazilian authority Anvisa:
Flavio Cadegiani , Erica M Lin , Andy Goren , Carlos G Wambier Potential risk for developing severe COVID-19 disease among anabolic steroid users BMJ Case Reports (2021) doi: 10.1136/bcr-2021-241572
BMJ editors are quoted by El Pais with:
What exactly is the BMJ investigating? The authors proudly admit they don’t believe in ethics approvals. Here Carlos Wambier, dermatology professor at Brown University in USA:
LikeLiked by 1 person
Pingback: Strano comitato – ocasapiens