A recent scoop by Science shook the world of neuroscience. NIH researcher Eliezer Masliah, head of the $2.6 billion Division of Neuroscience at the National Institute on Aging (NIA) was exposed as a massive fraud. NIH then “made findings of research misconduct” for “falsification and/or fabrication involving re-use and relabel of figure panels”. The story is just beginning to unfold.
The basis of that was a 300-page dossier of 132 research papers Masliah published between 1997 and 2023. What Science didn’t get quite right: the lion share of that dossier was prepared by the data integrity sleuth Mu Yang, mouse behavioural scientist at Columbia University in USA.

The hornets’ nest of Cerebrolysin. Masliah, EVER Pharma, and the many connecting dots and interesting characters
By Mu Yang
Cerebrolysin is a very old drug. Half a century to be exact. Despite of the general lackluster interest from the scientific community —– only 599 papers have been published on it in since 1973 — somehow this substance made from pig brain tissue has been a magnet to shady players in science. Despite of these folks, or maybe we should say because of these folks (?), cerebrolysin’s sleek website features a mentally sharp older gentleman playing chess—- presumable a recovered patient. According to the Austrian manufacturer EVER Pharma, cerebrolysin could potentially help with stroke, dementia, traumatic brain injury (TBI), and cognitive impairment.
According to preclinical data from the US neuroscientist Eliezer Masliah, cerebrolysin “decreases amyloid-beta production” in a mouse model of Alzheimer’s“ (Rockenstein et al 2006), has neurotrophic effects in a mouse model of Rett syndrom (Doppler et al 2008), neuroprotective effects against neuropathology in the APP transgenic mouse model of AD (Ubhi et al 2009), neuroprotective effects in a mouse model of temporal tauopathies (Rockenstein et al 2014), neurogenic effects in a AβPP mouse model (Rockenstein et al 2011), efficacy in a mouse model of tauopathy (a neurodegenerative disease characterized by tau accumulation (Rockenstein et al 2014), modulates nerve growth factors in a mouse model of AD (Ubhi et al 2013), and improves the survival of neural stem cell grafts in an APP model of AD (Rockenstein et al 2015).
Together, these pieces of exciting evidence portray cerebrolysin as a promising agent in the fight against a number of neurodegenerative diseases. Compared to the lofty sounding titles of Masliah papers, the work on cerebrolysin by the Sweden-based researcher Hari Shanker Sharma (often co-authored with his Romanian colleague Dafin Muresanu) sounds more like discombobulated sci-fi novels from the 1950s. E.g., “Neuroprotective effects of cerebrolysin, a combination of different active fragments of neurotrophic factors and peptides on the whole body hyperthermia-induced neurotoxicity: modulatory roles of co-morbidity factors and nanoparticle intoxication” (Sharma et al 2012).
At the time of writing, 8 out of 21 papers on cerebrolysin by Masliah are under investigation; 25 out of 39 Sharma papers/chapters on cerebrolysin have been flagged on PubPeer, and 5 have already been retracted/removed. Three chapters on cerebrolysin were retracted along with the entire book that Hari Shanker Sharma and his wife and fellow University of Uppsala researcher Aruna Sharma wrote and edited (“edit” is a euphemism here).
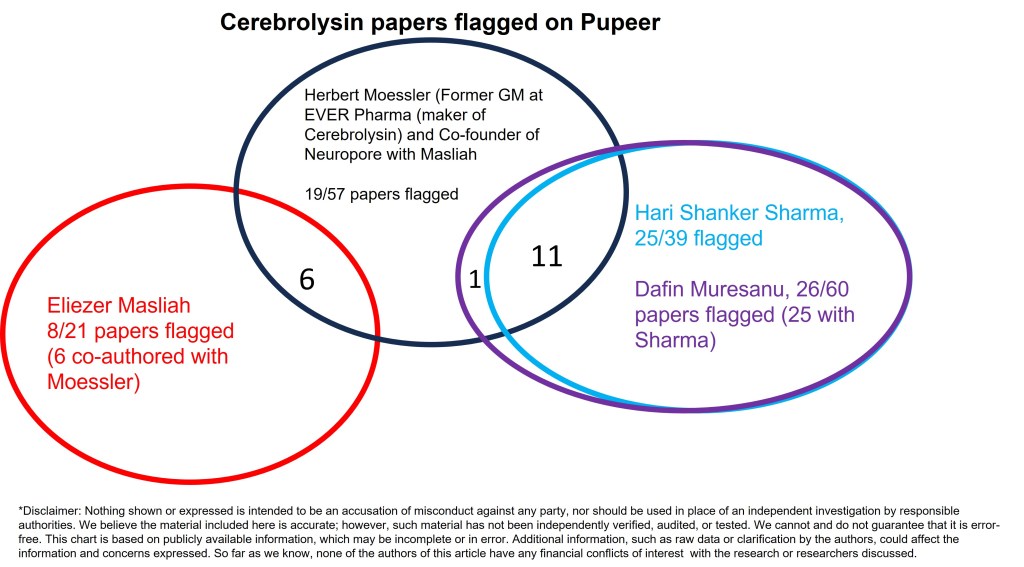
Below is a story how I discovered a lot of red flags in Eliezer Masliah’s papers, and how I followed the trace left behind by his Austrian business partner Herbert Moessler (former general manager at EVER Pharma, maker of Cerebrolysin) and tracked down the many questionable papers by Hari Shanker Sharma, including 25 on cerebrolysin. Both Masliah and the Sharmas now find themselves in hot water, and it is time for this Beijing-born behavioral scientist to share her story of busting bad papers that are completely outside her field of expertise.
In bed with Hari and Aruna
Hari Shanker & Aruna, a YouTube influencer couple in Sweden. With or without Rudolph the Red-Faced Liar. And with Anca and Dafin, two totally innocent and upright Romanians. Pushing pig brain juice an SS Nazi invented. You won’t find a better story for Christmas!
Serendipitous encounter with an old case revealed a new target
It began in September 2023. I had a little break from working on multiple Alzheimer’s cases in collaboration with the Science journalist Charles Piller and my teammates. Poking around PubMed (Dysdera arabisenen the spider is always on the hunt for new hornet’s nests) using various keyword combinations such as “Alzheimer’s therapeutics” “Alzheimer’s mouse model rescue” and the alike, I came across one image in two papers by Eliezer Masliah.
- Kiren Ubhi , Chandra Inglis , Michael Mante , Christina Patrick , Anthony Adame , Brian Spencer , Edward Rockenstein, Verena May , Juergen Winkler, Eliezer Masliah Fluoxetine ameliorates behavioral and neuropathological deficits in a transgenic model mouse of α-synucleinopathy Experimental Neurology (2012) doi: 10.1016/j.expneurol.2012.01.008
- Paula Desplats, Pruthul Patel , Kori Kosberg , Michael Mante , Christina Patrick , Edward Rockenstein , Masayo Fujita , Makoto Hashimoto , Eliezer Masliah Combined exposure to Maneb and Paraquat alters transcriptional regulation of neurogenesis-related genes in mice models of Parkinson’s disease Molecular Neurodegeneration (2012) doi: 10.1186/1750-1326-7-49

Something about this made me think that this was less innocent than a simple mistake. The image was used to indicate control mice with saline treatment in the Ubhi et al 2012 paper, but somehow the same image was used to indicate mice with a combination treatment of Maneb and Paraquat. Then, another paper came out of the ImageTwin scan with huge red flags. In this paper, images of three different mouse models overlap. Intriguingly, the Line 29 image seems to have been made to appear lighter. This screams manipulation to me.
Clifford W Shults , Edward Rockenstein , Leslie Crews , Anthony Adame , Michael Mante , Gabriel Larrea , Makoto Hashimoto , David Song , Takeshi Iwatsubo , Kyoko Tsuboi , Eliezer Masliah Neurological and neurodegenerative alterations in a transgenic mouse model expressing human alpha-synuclein under oligodendrocyte promoter: implications for multiple system atrophy The Journal of Neuroscience (2005) doi: 10.1523/jneurosci.3527-05.2005

The name of Masliah started to ring a bell. Searching what had been flagged on PubPeer, I realized I myself had flagged a few papers from the group almost a year prior, here and here and here. And so did Kevin Patrick and Elisabeth Bik, as this site had published earlier in 2023.
The potential problems of Eliezer Masliah
“the confusion occurred while utilizing prior panels as example ” – emeritus professor Eliezer Masliah
These pieces of evidence were enough to convince me that something was happening on a large scale. In my renewed effort on this old case, I flagged two studies on cerebrolysin, here and here . In the Rockenstein et al 2015 BMC Neuroscience study, the same images were used to represent control mice with and without cerebrolysin treatment, raising the question on what kind of experiments were actually done.
Edward Rockenstein, Kiren Ubhi, Michael Mante, Jazmin Florio, Anthony Adame , Stefan Winter , Hemma Brandstaetter, Dieter Meier, Eliezer Masliah Neuroprotective effects of Cerebrolysin in triple repeat Tau transgenic model of Pick’s disease and fronto-temporal tauopathies BMC Neuroscience (2015) doi: 10.1186/s12868-015-0218-7

Many potential therapeutic effects of cerebrolysin touted in Masliah’s studies were called into question during our investigation. Our team ended up finding potential fraud in 8 out of 21 cerebrolysin studies, spanning a decade.
Kiren Ubhi , Edward Rockenstein, Edith Doppler , Michael Mante , Anthony Adame, Christina Patrick, Margarita Trejo , Leslie Crews, Amy Paulino , Herbert Moessler , Eliezer Masliah Neurofibrillary and neurodegenerative pathology in APP-transgenic mice injected with AAV2-mutant TAU: neuroprotective effects of Cerebrolysin Acta Neuropathologica (2009) doi: 10.1007/s00401-009-0505-4


As in many other papers, a salient issue is duplicated lanes in Western blot images as such this study flagged by Dr. Elisabeth Bik and this one flagged by me initially.
Edward Rockenstein , Magdalena Torrance , Michael Mante , Anthony Adame , Amy Paulino , John B Rose , Leslie Crews , Herbert Moessler, Eliezer Masliah Cerebrolysin decreases amyloid-beta production by regulating amyloid protein precursor maturation in a transgenic model of Alzheimer’s disease Journal of Neuroscience Research (2006) doi: 10.1002/jnr.20818
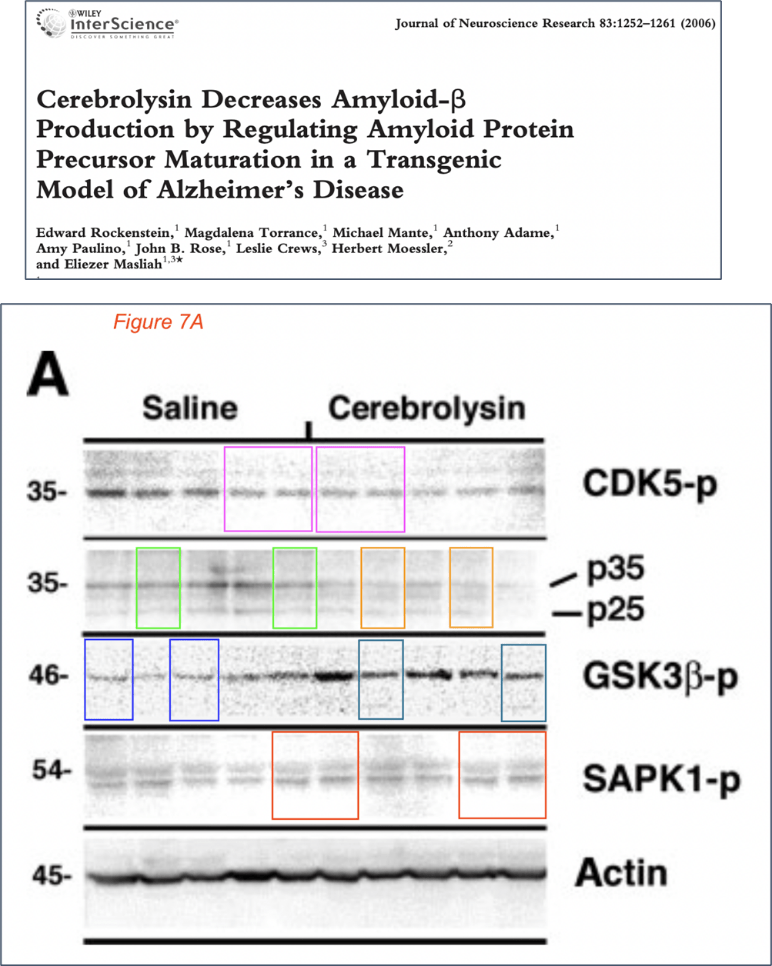
Image manipulation is another common artistic touch in Masliah’s papers. In the Rockenstein et al., 2011 paper, the histology image of a AβPP transgenic mouse (Alzheimer’s Disease (AD) model) treated with cerebrolysin bares striking resemblance to a control mouse image in the Crews et al, 2010 paper. Curiously, the AD mouse image has two blacks clumps (red circle) that are not in the control mouse image, raising the concerns on image manipulation.
Leslie Crews, Edward Rockenstein, Eliezer Masliah APP transgenic modeling of Alzheimer’s disease: mechanisms of neurodegeneration and aberrant neurogenesis Brain Structure and Function (2010) doi: 10.1007/s00429-009-0232-6

Similarly, an image AD mouse treated with cerebrolysin in Fig 3 of the Rockenstein et al 2011 study and an image of a control mouse in the Crews et al 2010 study overlap, leading to the concerns on the validity of both studies.

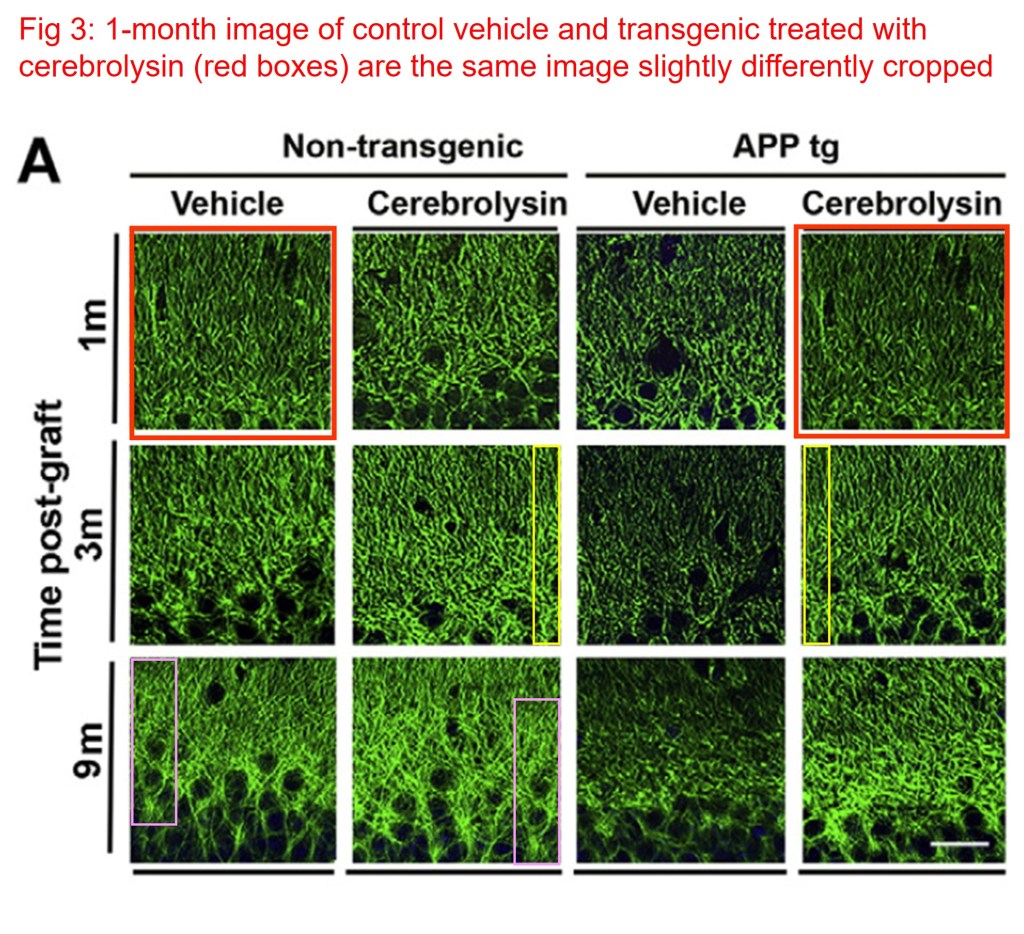
The single most flagged cerebrolysin study was the one I found in 2023. An image from the Spencer et al 2016 paper and one from Rockenstein et al., 2015 are strikingly similar, except for the region marked with blue trace.
Brian Spencer, Rewati Potkar , Jeff Metcalf , Ivy Thrin , Anthony Adame, Edward Rockenstein, Eliezer Masliah Systemic Central Nervous System (CNS)-targeted Delivery of Neuropeptide Y (NPY) Reduces Neurodegeneration and Increases Neural Precursor Cell Proliferation in a Mouse Model of Alzheimer Disease Journal of Biological Chemistry (2016) doi: 10.1074/jbc.m115.678185

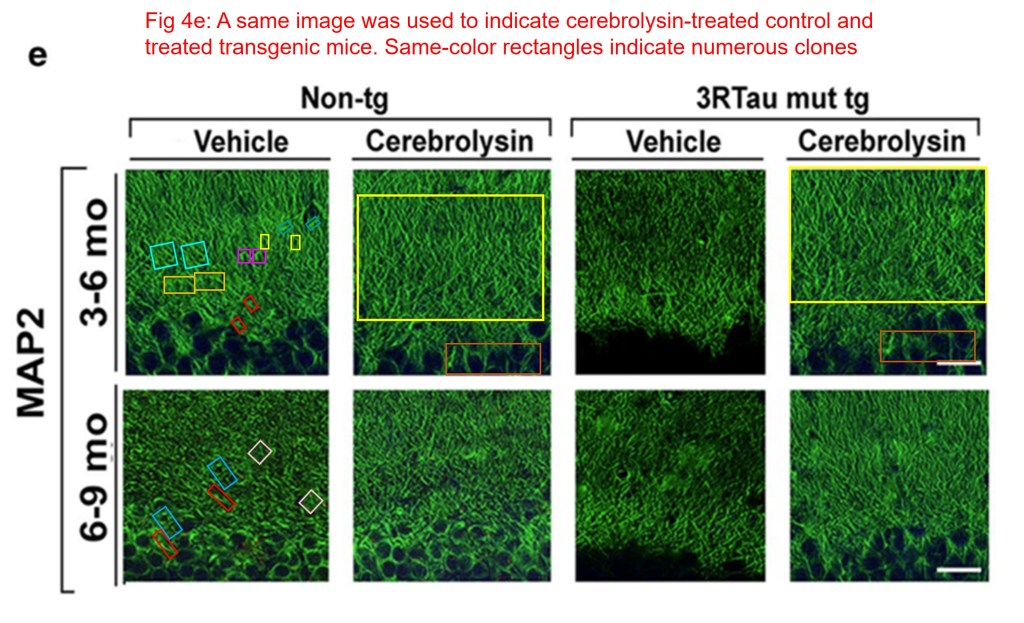
A large number of clones were found in Fig 4e by Kevin Patrick, also known as Cheshire.
In addition, Dr. Bik flagged issues in Western blot in this Rockenstein et al 2015 paper:

“Figure 3: With the orange-boxed marked duplications, the band-of-interest might have been changed but the backgrounds look remarkably similar. Boxes of the same color highlight blocks of lanes that look remarkably similar”
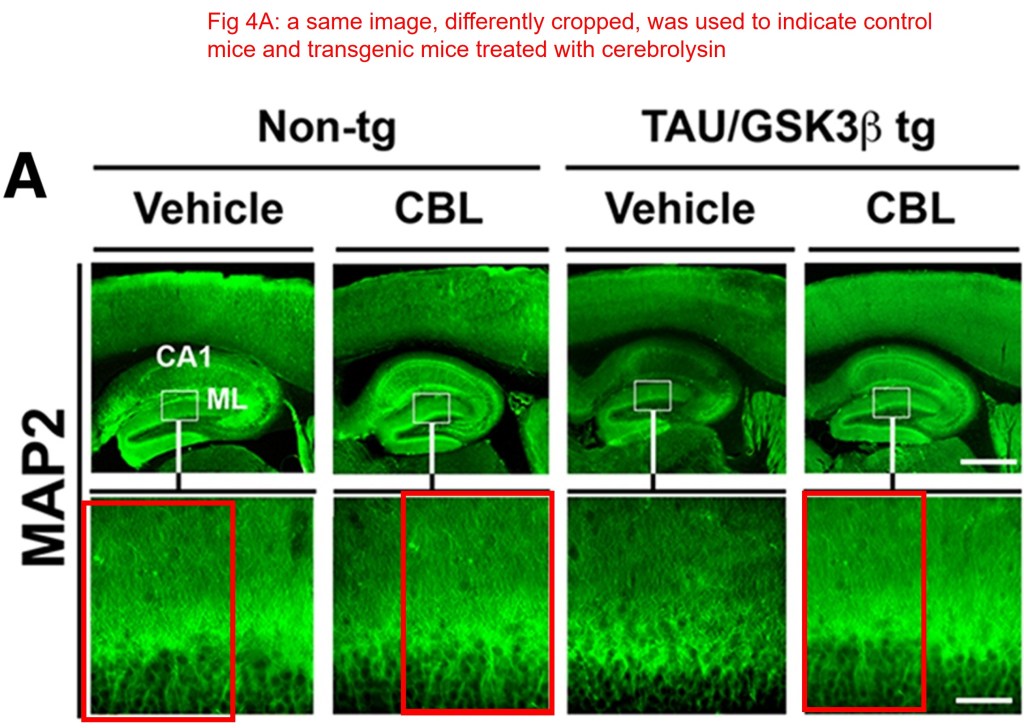
In this paper, the same image was used to indicate control mice and transgenic mice treated with cerebrolysin, misleading the readers on the “therapeutic effects” of the drug.
Edward Rockenstein , Paula Desplats , Kiren Ubhi , Michael Mante , Jazmin Florio , Anthony Adame , Stefan Winter , Hemma Brandstaetter , Dieter Meier , Eliezer Masliah Neuro-peptide treatment with Cerebrolysin improves the survival of neural stem cell grafts in an APP transgenic model of Alzheimer disease Stem Cell Research (2015) doi: 10.1016/j.scr.2015.04.008
Similarly, in this paper , we also saw a same image being used to indicate control mice and transgenic mice treated with cerebrolysin. The brilliant “treatment effects” seem to be the result of a thoughtful arrangement of stock images.
Edward Rockenstein , Kiren Ubhi , Margarita Trejo , Michael Mante , Christina Patrick , Anthony Adame , Philipp Novak , Marion Jech , Edith Doppler , Herbert Moessler , Eliezer Masliah Cerebrolysin™ efficacy in a transgenic model of tauopathy: role in regulation of mitochondrial structure BMC Neuroscience (2014) doi: 10.1186/1471-2202-15-90
In January 2024, to ensure that Charles Piller’s book project moved forward on time, the team had called it a stop on the Masliah case. Just starting to grasp the artistic style of this group, I pleaded to have the deadline extended so that I could do another round of deep combing. The final round turned into three. It was during this solo fight period when I found numerous within-image clones in many Masliah studies, including the ones in Fig 1C of the Rockenstein et al 2015 study.

Other examples of image clones that I found can be found in Rockenstein et al 2014, Spencer et al 2016, or in Bilal Fares et al 2023. Or this, a collaboration by Masliah with Stanford neurologist Tony Wyss-Coray:
Fiona Pickford , Eliezer Masliah , Markus Britschgi , Kurt Lucin , Ramya Narasimhan , Philipp A. Jaeger , Scott Small , Brian Spencer , Edward Rockenstein , Beth Levine , Tony Wyss-Coray The autophagy-related protein beclin 1 shows reduced expression in early Alzheimer disease and regulates amyloid β accumulation in mice Journal of Clinical Investigation (2008) doi: 10.1172/jci33585

By a conservative count, I contributed to about 160 out of 300 slides in the final dossier —- about 70 slides were done by me during the final solo deep combing. Here is Masliah’s collaboration with Robert Rissman, neuroscientist at the University of Southern California:
Omar El-Agnaf , Cassia Overk , Edward Rockenstein , Michael Mante , Jazmin Florio , Anthony Adame , Nishant Vaikath , Nour Majbour , Seung-Jae Lee , Changyoun Kim , Eliezer Masliah , Robert A. Rissman Differential effects of immunotherapy with antibodies targeting α-synuclein oligomers and fibrils in a transgenic model of synucleinopathy Neurobiology of Disease (2017) doi: 10.1016/j.nbd.2017.05.002

Discoveries on a rainy day
Compared to the disappointing false promises in Masliah’s flagged cerebrolysin studies, Hari Shanker Sharma, with his long-term collaborator Dafin Muresanu, portrayed a fantasy land in which cerebrolysin was a magical all-purpose neuroprotective agent for Alzheimer’s, Parkinson’s, concussive brain injury, heat stroke, spinal cord injury, often in very odd combinations:
Aruna Sharma , Dafin F. Muresanu , Rudy J. Castellani, Ala Nozari , José Vicente Lafuente , Seaab Sahib , Z. Ryan Tian , Anca D. Buzoianu , Ranjana Patnaik , Lars Wiklund , Hari Shanker Sharma Mild traumatic brain injury exacerbates Parkinson’s disease induced hemeoxygenase-2 expression and brain pathology: Neuroprotective effects of co-administration of TiO2 nanowired mesenchymal stem cells and cerebrolysin Progress in brain research (2020) doi: 10.1016/bs.pbr.2020.09.010

Who are the Sharmas? According to Dove Press “Dr. Hari Shanker Sharma is Professor of Neurobiology (MRC) and Docent in Neuroanatomy (UU) at the Department of Surgical Sciences, Uppsala University Hospital (Sweden)”. I discovered the Hari Shanker Sharma and his wife Aruna in early December, 2023, on a very rainy day that kept me home. Since I already flagged several cerebrolysin papers by Masliah, I started looking into other preclinical cerebrolysin studies on PubMed.

Quickly, I tracked down a book published by Springer. Realizing that the two editors were authors on every single chapter in this book, I started to scan the images in the book with ImageTwin. The evidence was so easy to find, that by 10:30 am, I had finished compiling a 12-slide report that led to the retraction of the entire book.
In my following search, I was astonished to find serious image and data issues in TEN books published by Elsevier that the Sharmas were editors for. This raises the question of whether “book series”, especially ones by Elsevier, are a convenient alternative to publishing peer-reviewed papers. Since each chapter in books edited by themselves appear on PubPeer as a regular review article, the Sharmas have a remarkable publication record. Back to cerebrolysin, the Sharmas seem to have a very unique technique of delivering cerebrolysin into the brain via a “nanowired” mechanism. Searching “nanowire delivery cerebrolysin” on PubMed, you will see that all six papers are by the Sharmas, and all are flagged by sleuths. Their artwork had been prominently featured in a previous article on this site, I will only show some new finds here. And the bottom line is, if 25 out of 39 Sharma’s papers on cerebrolysin have been flagged, is that just a matter of time and effort that we find significant problems in the rest?
A same image was used to illustrated 4 different experimental drug treatment conditions in four articles — 3 out of 4 are on cerebrolysin.
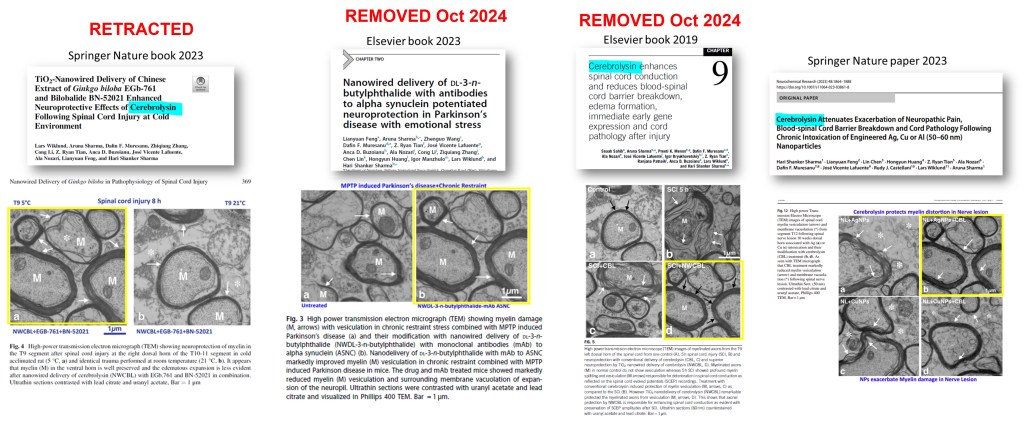
Ozkizilcik et al 2018 and Ozkizilcik et al 2019 contain the same images (labeled differently). Smut Clyde found the duplications between papers (AI didn’t find it, Smut found it based on memory!) after I pointed to the pasted-in upper left corners in the image from the 2019 paper.
- Asya Ozkizilcik , Aruna Sharma , Dafin F. Muresanu , José V. Lafuente , Z. Ryan Tian , Ranjana Patnaik , Herbert Mössler , Hari S. Sharma Timed Release of Cerebrolysin Using Drug-Loaded Titanate Nanospheres Reduces Brain Pathology and Improves Behavioral Functions in Parkinson’s Disease Molecular Neurobiology (2018) doi: 10.1007/s12035-017-0747-4
- Asya Ozkizilcik , Aruna Sharma , José Vicente Lafuente , Dafin F Muresanu , Rudy J Castellani , Ala Nozari , Z Ryan Tian , Herbert Mössler , Hari Shanker Sharma Nanodelivery of cerebrolysin reduces pathophysiology of Parkinson’s disease Progress in brain research (2019) doi: 10.1016/bs.pbr.2019.03.014
In addition to image tricks, data in Sharma’s papers often look puzzling. in Table 1 in Sharma et al 2019, we see 0.23 occurring 10 times, and 0.34 for 11 times.
Aruna Sharma , Dafin F. Muresanu , Asya Ozkizilcik , Z. Ryan Tian , José Vicente Lafuente , Igor Manzhulo , Herbert Mössler , Hari Shanker Sharma Sleep deprivation exacerbates concussive head injury induced brain pathology: Neuroprotective effects of nanowired delivery of cerebrolysin with α-melanocyte-stimulating hormone Progress in brain research (2019) doi: 10.1016/bs.pbr.2019.03.002

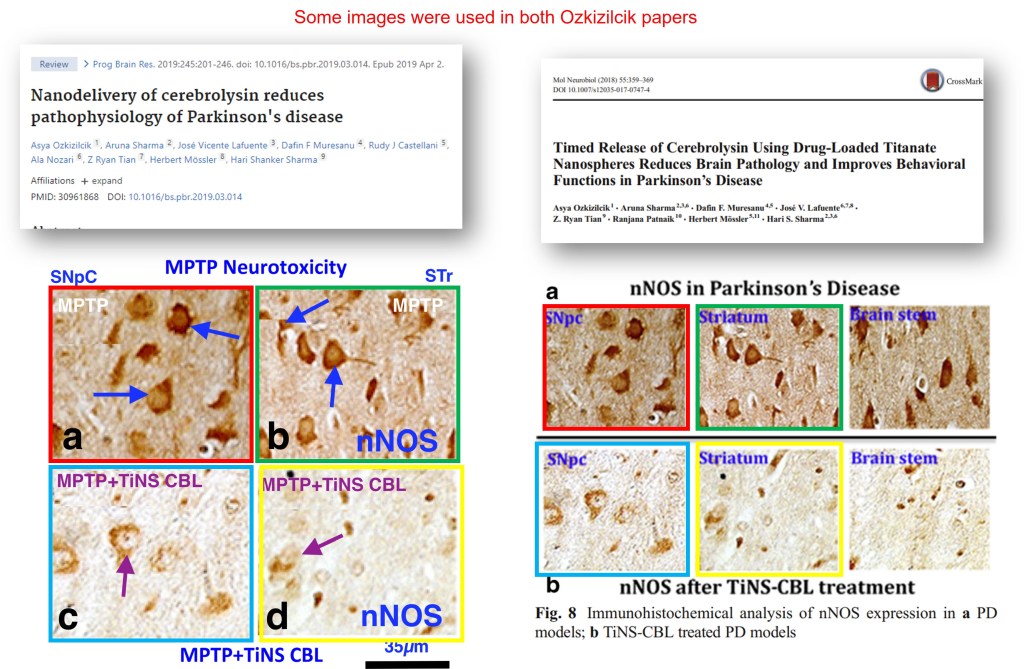
Similarly, in a study on “PLGA nanoparticles loaded cerebrolysin” (Rouzi et al 2015), 0.08 was a very popular SD, occurring 17 times in Table 2.
Barbara Ruozi , Daniela Belletti , Hari S. Sharma , Aruna Sharma , Dafin F. Muresanu , Herbert Mössler , Flavio Forni , Maria Angela Vandelli , Giovanni Tosi PLGA Nanoparticles Loaded Cerebrolysin: Studies on Their Preparation and Investigation of the Effect of Storage and Serum Stability with Reference to Traumatic Brain Injury Molecular Neurobiology (2015) doi: 10.1007/s12035-015-9235-x
Lastly, it is worth noting that the Sharmas always clearly credit EVER Pharma for supporting their work in their presentations. Here are two examples. One is a poster presentation on cerebrolysin in 2015, and another is a talk on cerebrolysin that Hari Sharma gave in 2016. There are numerous uploads of conference clips that the Sharmas uploaded on YouTube.
What I find puzzling is that Dafin Muresanu, who has 60 papers on cerebrolysin (including 7 clinical trials) since 2008, didn’t know better. On the website of World Federation of Neurorehabilitation, Muresanu was described as “Professor of Neurology, MD, Ph.D., MBA, Chairman of the Neurosciences Department, University of Medicine and Pharmacy, Cluj-Napoca, Romania, President of the European Federation of Neurorehabilitation Societies, Corresponding Member of the Romanian Academy“. Muresanu was on all 25 of Sharma’s cerebrolysin papers flagged by sleuths. In addition to publishing with the Sharmas, Dr. Muresanu also co-authored a paper with Herbert Moessler at EVER Pharma, on a human study. Interestingly, Muresanu is also the Editor-in-Chief of Journal of Medicine and Life where the paper was published:
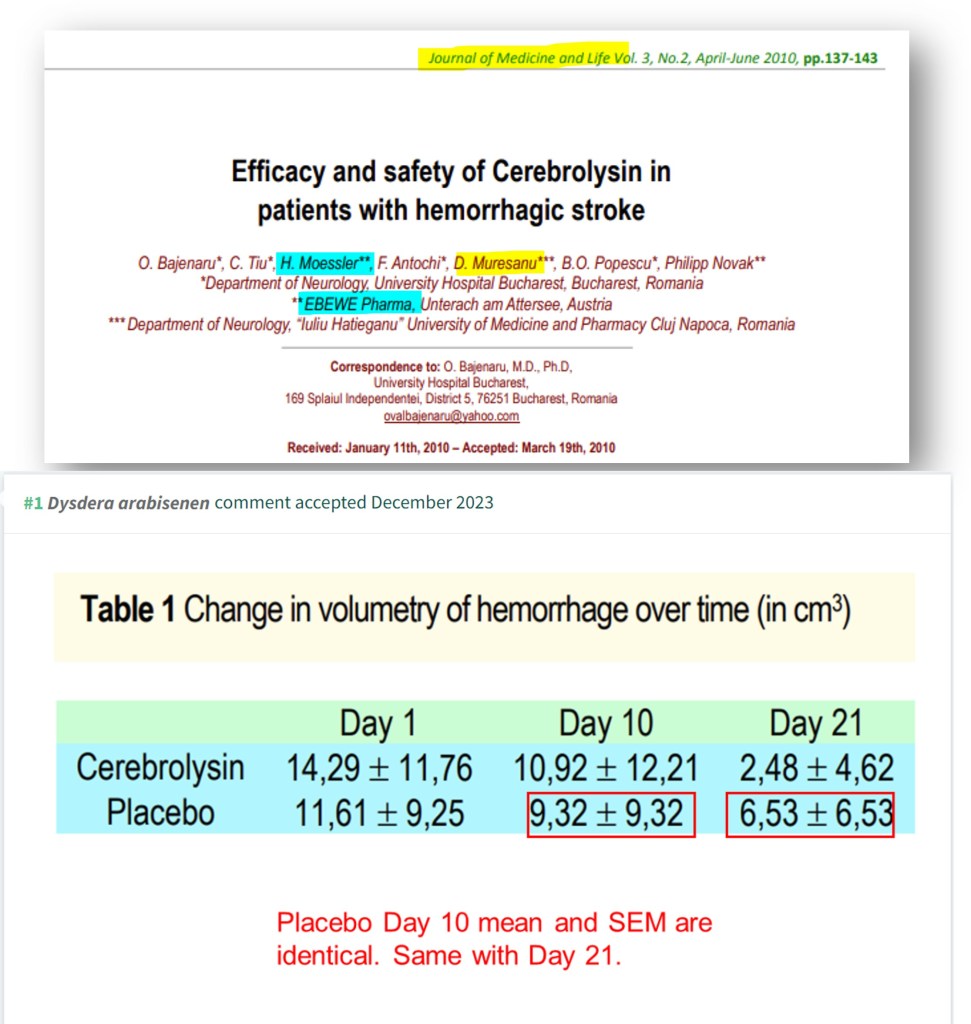
O Bajenaru, C Tiu , H Moessler , F Antochi , D Muresanu , B O Popescu , Philipp Novak Efficacy and safety of Cerebrolysin in patients with hemorrhagic stroke Journal of Medicine and Life (2010) PMCID: PMC3019043
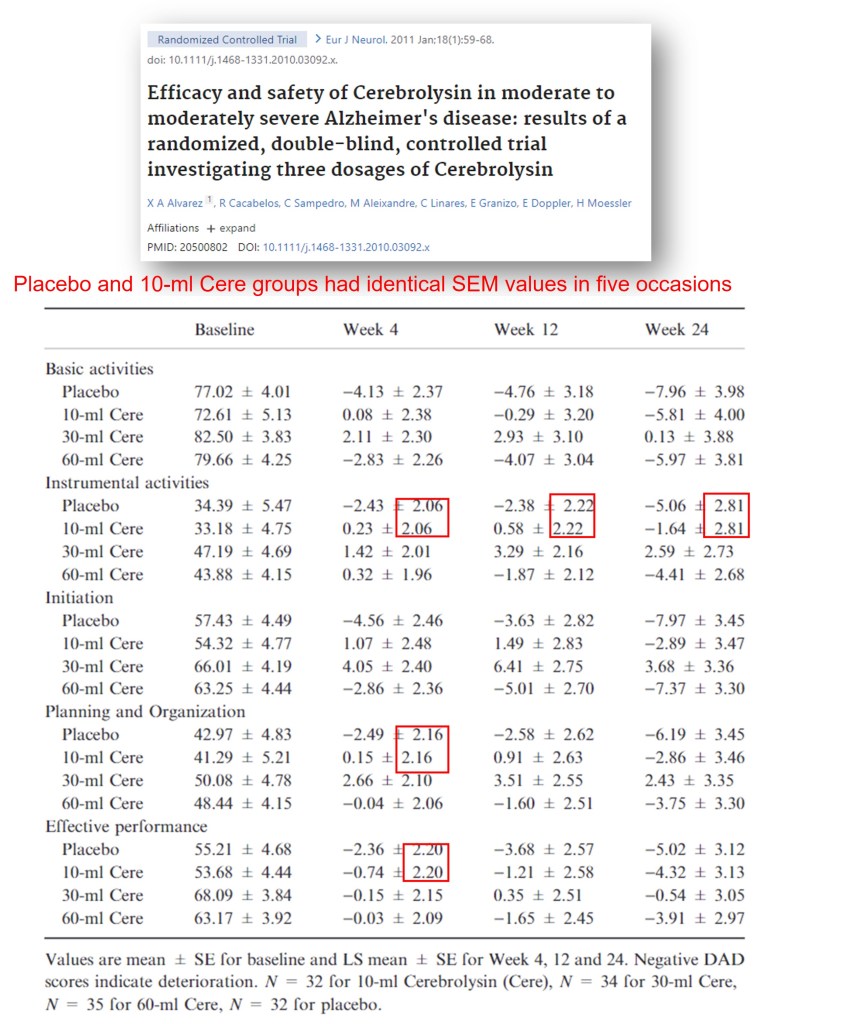
Speaking of Herbert Mössler, as a co-author on 57 papers on cerebrolysin (15 under Mössler and 42 under Moessler), he should know the stuff well. But, Mössler co-authored 11 questionable cerebrolysin papers with the Sharmas. How are Mössler’s papers without the Sharmas? In a clinical trial (Alvarez et al 2011), control and 10-mil cerebrolysin groups have the same SEM values (and very different means) on five occasions.
X A Alvarez, R Cacabelos , C Sampedro , M Aleixandre , C Linares , E Granizo , E Doppler , H Moessler Efficacy and safety of Cerebrolysin in moderate to moderately severe Alzheimer’s disease: results of a randomized, double-blind, controlled trial investigating three dosages of Cerebrolysin European Journal of Neurology (2011) doi: 10.1111/j.1468-1331.2010.03092.x
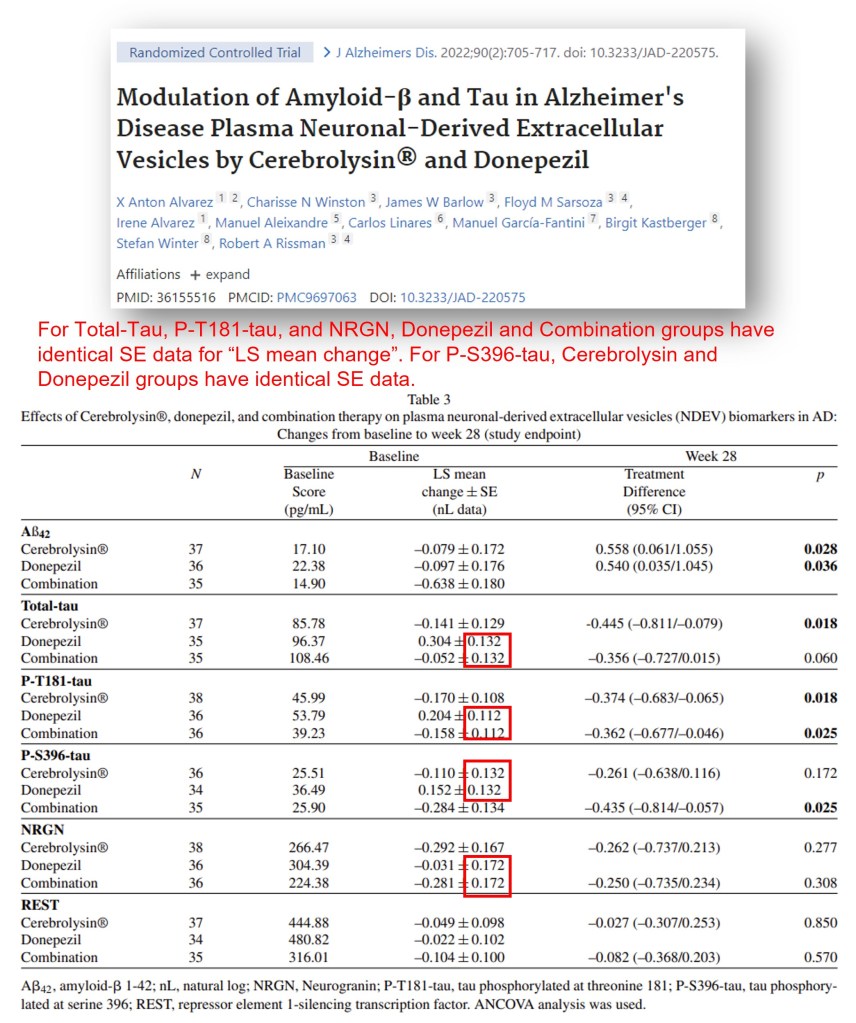
Is this an isolated mistake? Well, in another Alvarez paper ELEVEN years later (Alvarez et al., 2022), we saw a similar incidence where different groups had the same SEM values on multiple occasions. The senior author of this paper was the aforementioned Californian neuroscientist Robert Rissman, senior author on 7 out of 16 papers that he published with Eliezer Masliah. According to Science, “USC says it will conduct a confidential review of Rissman’s involvement with Masliah’s work.“. Do we think that he read this manuscript on drug effects in HUMAN subjects carefully?
X Anton Alvarez , Charisse N Winston , James W Barlow , Floyd M Sarsoza , Irene Alvarez , Manuel Aleixandre , Carlos Linares , Manuel García-Fantini , Birgit Kastberger , Stefan Winter , Robert A Rissman Modulation of Amyloid-β and Tau in Alzheimer’s Disease Plasma Neuronal-Derived Extracellular Vesicles by Cerebrolysin® and Donepezil Journal of Alzheimer’s Disease (2022) doi: 10.3233/jad-220575
Torrid water
How did a silly-sounding old-school drug got such a bad name? Let’s look at how it all started.

Cerebrolysin seems to fall into the “holistic medicine” category. In Chinese, we have a saying “以形補形” which means something like “to heal an organ (or a body part) by eating the corresponding organ (or a body part) of an animal, or something similar in shape or colour“. Did I mention that cerebrolysin is made from pig’s brain?
The drug has a particularly benevolent-sounding in Hong Kong — 施普善 which translates loosely to “to share/spread kindness broadly“. It doesn’t seem outrageous for people to conceive therapeutic effects of something widely available and probably cheap in healing the ills in the 1970s, or in relatively under-developed countries. But it does seem odd that Masliah, with all the modern medicines available to him, would be so into cerebrolysin for nearly two decades. We do know that Masliah received cerebrolysin from EVER Pharma in the 1990s, as indicated in Masliah et al., 1999.
Moessler’s first paper on cerebrolysin dates to 1997, it was a clinical trial on 645 Austrian patients suffering from dementia (Rainer et al 1997). Masliah started to publish with Moessler on cerebrolysin in 2002 (Rockenstein et al 2022). In the same year, Masliah also started to publish with Manfred Windisch, who until 1999 was the CEO of the Austrian company EBEWE Pharma, which until 2008 owned the cerebrolysin-maker EVER Pharma. In 2000, Windisch edited the Springer book “Advances in Dementia Research“, its last chapters are dedicated to cerebrolysin. One of those was contributed by Masliah, two by Windisch with his Spanish associate Xose Anton Alvarez (who later worked with Rissman):

Windisch’s last collaboration with Masliah was in 2013, but Moessler continued. He however kept separate his collaborations with Masliah on one side and with Muresanu (and Sharmas) on the other side (except for one study, Alvarez et al 2016).
Moessler held senior management positions at EVER Pharma from 2006 to 2015. In 2008, he co-founded on behalf of EVER Pharma the California-based biotech Neuropore Therapies with Masliah and Masliah’s then-employer, the University of California San Diego.
Was cerebrolysin really that interesting to Masliah that he published 21 papers on it, or is there something else? As we can see from the timeline, the first cerebrolysin study found to contain serious issues was by Masliah (Rockenstein et al., 2006). In the following years, a number of studies were published by Masliah, Moessler, Muresanu, and Sharma, and were later flagged by sleuths. According to our record, it wasn’t until 2013 that other small players started to publish fraudulent papers on cerebrolysin. The timeline suggests that the “hopes and promises” in the “body of literature” established by the main players from the 1990s to early 2010s paved the way for more fraudulent papers to occur. Prior to late 1990s, cerebrolysin had a quiet, boring history. Interests started to pick up in late 1990s, around the time when the prominent American neuroscientist Masliah started to publish on it. Below are some studies by followers of cerebrolysin.
Abdel-Salam et al 2013, flagged by Kevin Patrick and myself, contain numerous image duplications:
Omar Abdel-Salam, E A Omara , N A Mohammed , E R Youness , Y A Khadrawy , A A Sleem Cerebrolysin attenuates cerebral and hepatic injury due to lipopolysaccharide in rats Drug Discoveries & Therapeutics (2013) doi: 10.5582/ddt.2013.v7.6.261

Two papers from China Liu et al., 2022 and Tao et al., 2021, contain identical Western blots with different labels.
- Weihong Lu , Zhonghua Zhu , Dongliang Shi , Xiaoyu Li , Jingzhi Luo , Xingzhi Liao Cerebrolysin alleviates early brain injury after traumatic brain injury by inhibiting neuroinflammation and apoptosis via TLR signaling pathway Acta cirurgica brasileira (2022) doi: 10.1590/acb370605
- Yunna Tao , Yeping Xu , Meng Shen , Xiaoyan Feng , Yan Wu , Youping Wu , Liuyan Shen , Yuhai Wang The neuroprotection of cerebrolysin after spontaneous intracerebral hemorrhage through regulates necroptosis via Akt/ GSK3β signaling pathway Acta cirurgica brasileira (2021) doi: 10.1590/acb361002

Abdel-Salam et al., 2014 flagged by Sholto David contains numerous cloned elements.
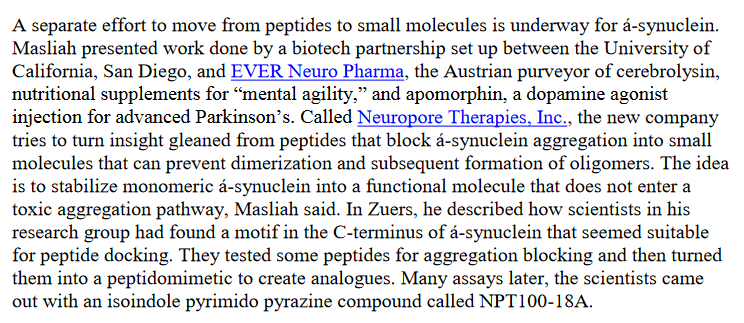
Omar Abdel-Salam, Nadia Mohammed , Eman Youness , Yasser Khadrawy , Enayat Omara , Amany Sleem Cerebrolysin protects against rotenone-induced oxidative stress and neurodegeneration Journal of Neurorestoratology (2014) doi: 10.2147/jn.s50114
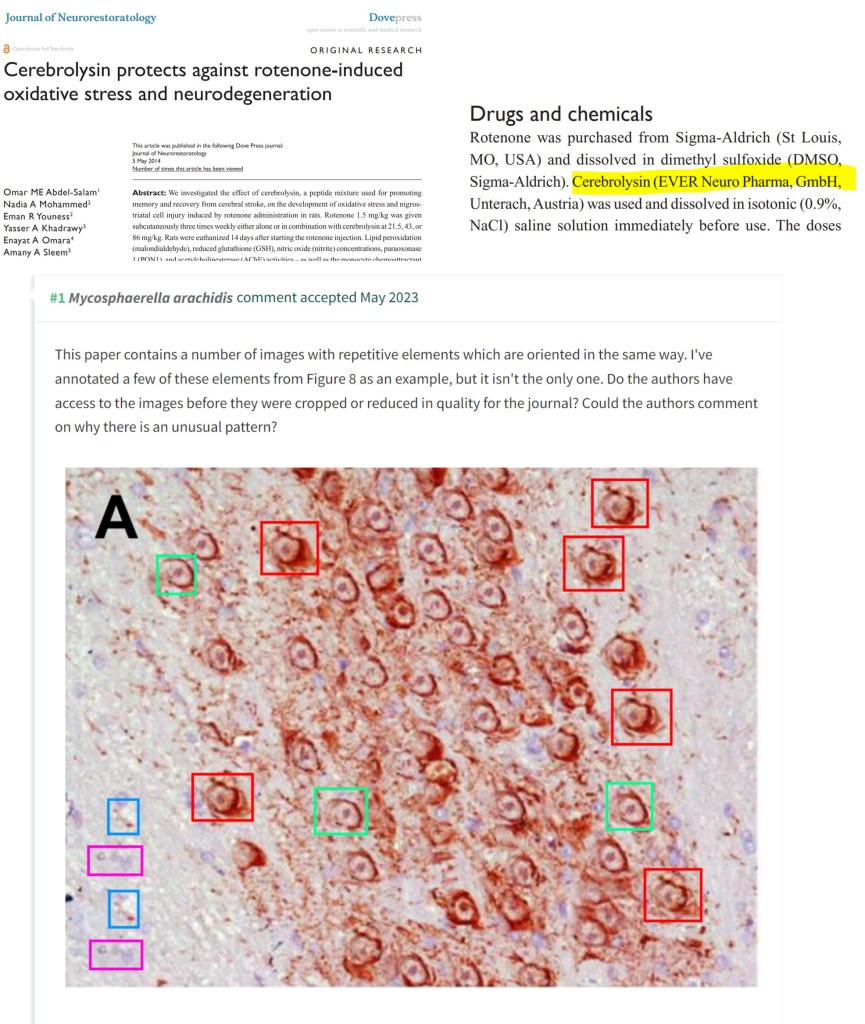
El-Marasy et al 2021 from Egypt contains image duplications.
Salma A El-Marasy , Sally A El Awdan , Azza Hassan , Omar A Ahmed-Farid , Hanan A Ogaly Anti-depressant effect of cerebrolysin in reserpine-induced depression in rats: Behavioral, biochemical, molecular and immunohistochemical evidence Chemico-Biological Interactions (2021) doi: 10.1016/j.cbi.2020.109329
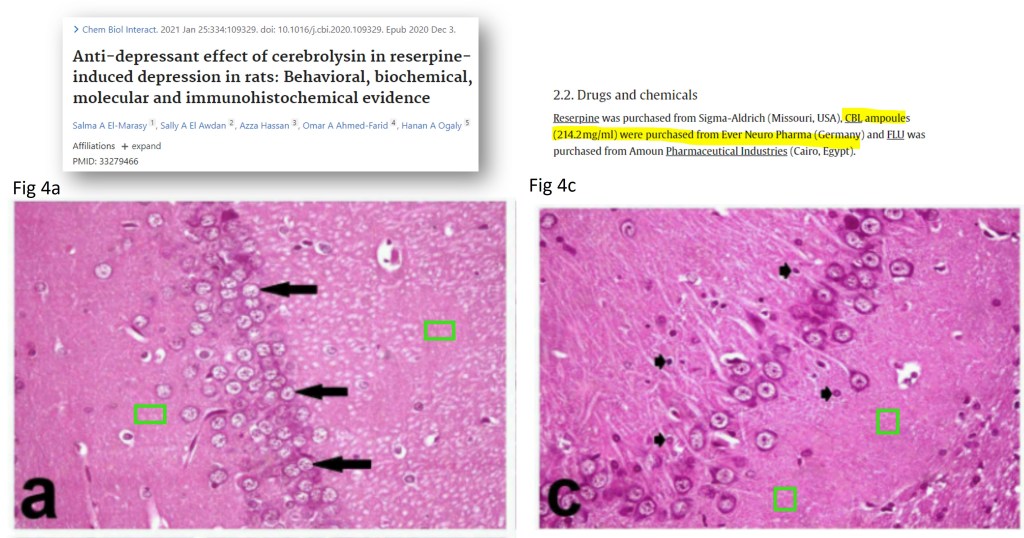
The Turkish study Tonge et al 2023, flagged by Rene Aquarius, contains image duplications.
Caghan Tonge , Pinar Kuru Bektasoglu, Ahmet Gulmez , M Erhan Turkoglu , Ata Turker Arikok , Berrin Imge Erguder , Bora Gurer , Hayri Kertmen Cerebrolysin Amelioration of Spinal Cord Ischemia/ Reperfusion Injury in Rabbit Model Turkish Neurosurgery (2023) doi: 10.5137/1019-5149.jtn.42362-22.6
Here is a list of Cerebrolysin papers with PubPeer links:
Take home message
Base on the timeline, I would speculate that Moessler introduced cerebrolysin to Masliah in the late 1990s and to Muresanu a few year later. According to his LinkedIn profile, Moessler held prominent positions in EVER Pharma between 2006 and 2015. Does this have anything to do with his success in introducing cerebrolysin to prominent researchers in Europe and the US? It is intriguing that he co-founded Neuropore with Masliah in 2008. I want to further speculate that Muresanu introduced cerebrolysin to Hari Shanker Sharma in late 2000s, and the two decided to publish almost every single cerebrolysin paper ever since (2010-2023). What is in it for these players? Your guess is as good as mine.
What is in it for the numerous elderly patients being given a glimmer of hope that a cure might be on the horizon though?
Donate!
If you are interested to support my work, you can leave here a small tip of $5. Or several of small tips, just increase the amount as you like (2x=€10; 5x=€25). Your generous patronage of my journalism will be most appreciated!
€5.00




Pubpeer allows both positive and negative comments.
LikeLike
99% of comments on PubPeer are negative.
LikeLike
Cerebrolysin, then, is essentially an injectable pig brain smoothie?
LikeLiked by 1 person
I call it Nazi Pig Brain Juice. Invented by Gerhard Harrer, member of SS and NSDAP, after WWII one of Austria’s most powerful neurologists and Nazi scum till his death. That’s why Ever Pharma owns the patent iand that’s why Nazi Pig Brain Juice is an approved therapy in Austria. It is banned in USA.
LikeLiked by 2 people
The worst part is that Dr. Masliah, being Jewish, was capable of ‘giving validity’ to that nonsense originating from Nazi times; it is truly despicable.
LikeLike
For such people, Nazi Pig Brain Juice is kosher.
But then again, Stephen Miller and Laura Loomer prove that these days also Jews can be Nazis.
LikeLike
I am disappointed that the Swedish Nobel Academy failed to recognize the major contributions by Chinese scientists to MicroRNA and their ability to cure all kinds of known and imagined diseases of mankind.
https://pubmed.ncbi.nlm.nih.gov/?term=microRNA++and+retraction
LikeLiked by 2 people
Oh my, never knew there was a thing called “Neurorestoratology“?!?!
LikeLike
My friend Greg Fitzgerald and I did a deep dive into Cerebrolysin recently. We had a simple question – what is in it? The pharma company claims it has neurotrophic peptides. But that flies in the face of common sense – first of all, most peptides degrade easily and require refrigeration or desiccation. Secondly, peptides don’t penetrate the BBB like Ever Pharma Claims. Finally, why should a lysate of pig brain contain therapeutic quantities of peptides?
It doesn’t make sense. In fact, Cerebrolysin was originally described as an amino acid mixture. Recent HPLC studies show it is mostly amino acids, salt, and protein fragments.
So there is no good mechanism by which it could have any of the therapeutic effects claimed! We knew the literature must be wrong, but we had no idea of the scope and breath of the fraud, nor the audacity of it.
This is really sad because Cerebrolysin is growing in popularity in the US after Bryan Johnson tried it, and it is widely used in many countries, especially for stroke and TBI recovery.
You can find our article on Substack here.
LikeLiked by 1 person
Hi Dan, thanks for commenting.
I just saw several of your comments ended up in spam. Sometimes WordPress does this, and I ask my readers to always notify me if their comments do not appear.
LikeLike
Thanks. Yeah my comment kept not going through — I tried three times. Then I came back two days later and tried posting again. I’ve added a link to this article in my article, by the way. It’s a tour de force piece of work.
LikeLiked by 2 people
Is infamous that these kind of cheating was made in detrimental of the health of the people and against all ethics. I was almost in one feet to enter to his lab as postdoc years ago, but I did not since I decided to go into another country, where I was accepted. Is really dissapointing for those who still believe in ethics on Science. Thanks for spread the voice, we have to be critics of the infamous.
LikeLike