The Italian sleuth Aneurus Inconstans is always at your service when Italian science superstars need to be celebrated, be it in Italy or in USA. Today’s hero is a US cancer researcher with Italian roots: Salvatore Pizzo, Distinguished Professor of Pathology at Duke University in North Carolina. He is also a Fellow of American Association for the Advancement of Science (AAAS) since 1999.
Pizzo is 80 years young and urgently need to retire both himself and his papers.
It is not just Pizzo’science which is questionable. There was this lawsuit Dodd vs Pizzo from 2002, where Leslie G. Dodd (now professor at University of North Carolina Chapel Hill) sued Pizzo and Duke University for discrimination, harassment and “intentional or negligent infliction of emotional distress against both Defendants and assault and battery against Defendant Pizzo only.”
Dodd was hired by Pizzo in 1993 and promoted in 1996, they began a sexual relationship which Dodd ended in 1997, but which Pizzo wanted to continue, allegedly offering Dodd “improved job conditions” in return. Dodd claimed Pizzo began retaliating against her, and she lost her position as as Division Chief of Cytology. She also gave to protocol that Pizzo had been “ordering an expensive item of equipment which she did not request over her forged signature, . . . generating expressions of ill will in the Department of Pathology against Plaintiff, causing other professors to refuse to work with her, and having her work subjected to uncalled for and unusual quality reviews.” The court decided with:
“Granted as to Plaintiff’s Title VII claims of hostile work environment, sex discrimination, and retaliation, but denied as to Plaintiff’s Title VII claim of quid pro quo sexual harassment”
And now, Aneurus will tell you about Sal Pizzo’s scientific activities. And not just his! There will also be Pizzo’s Canadian collaborator Rick Austin, with bonus other Duke researchers and their collaborators.
Top Italian Scientists
“You may think this is just a silly prank with zero impact on whatsoever, but no. […] this initiative is useful for something. It provides solid numbers for quantifying the extent of scientific misconduct in Italy and beyond” – Aneurus Inconstans
Cancer at Duke? Better call Sal!
By Aneurus Inconstans
Academia is breaking bad. When neither controls nor controllers are in place, any human enterprise soon turns into a circus or worse, and academia is no exception. Over the years the public got educated on the importance of research for society and human health, without knowing that academic research is often ruled by overgrown kids who play scientists.
The main protagonist of today’s story (although not alone) is an octogenarian boy at Duke University Medical Center (DUMC), who churns out fake cancer research for twenty years. Please meet Salvatore Vincent Pizzo, whom we will amicably call “Sal” hereafter, distinguished professor at the Department of Pathology of the Duke University School of Medicine, who sports around 35 articles tagged on PubPeer for outrageously faked data.
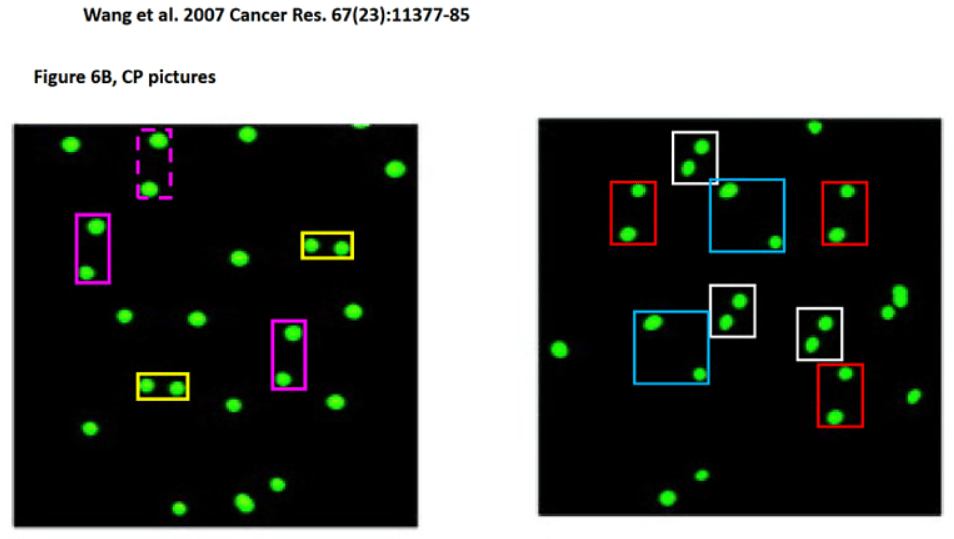
Below is a twenty-year-old study, flagged by our sleuth colleague Clare Francis, where the offending blots are describing at the same time p-eIF2α and Akt, or the trio GRP78, XBP-1 and GADD34, or p-eIF2α and p-Akt, or the quintet GRP78, p-ERK, p-eIF2α, p-FKHR and p-Akt, plus two actin controls reused for different conditions. The article is supposed to show that insulin-dependent mechanisms overcome the insulin-induced ER stress by up-regulating the unfolded protein response (UPR) and the anti-apoptotic pathway to promote cell survival. Look yourself if we can trust the outcome:
Uma Kant Misra, Salvatore Vincent Pizzo* Up-regulation of GRP78 and antiapoptotic signaling in murine peritoneal macrophages exposed to insulin Journal of Leukocyte Biology (2005) doi: 10.1189/jlb.1104685




The myriad of blots published in that scientific atrocity illustrated above come from the ‘hard work’ of just two authors: Sal himself and his former Duke colleague and distinguished emeritus professor Uma Kant Misra (26 papers on PubPeer). At the time of this 2005 work, Sal was aged 60 and Uma 80. Yes indeed. Please read below Sal’s words to Leonid (highlights mine):
“I have NEVER faked data. I tried to locate Uma but haven’t been able to reach him. Uma would be well over 100 and I believe he has passed on. The data shown in our papers was always from single gels unless otherwise stated.”
Sal Pizzo
Were these two old professors really pipetting day and night all alone? No students or postdocs to help? No-one to shift blame to?
We all hope Uma is still with us and maybe Sal will eventually find him somewhere. Meanwhile, although unable to reach Uma, Sal decided to defend his old friend (and himself) unconditionally, and threatened legal actions:
“We have never done this. If you wish to carry on what appears to be a vendetta please supply me the name of your lawyer and I will have my lawyer contact him.”
Sal Pizzo
Below is another reprehensible vendetta, whose target is a more recent production (2017) published in that trash can called Oncotarget. This time Dr. Misra is not on board, the sole author other than Sal is a certain Udhayakumar Gopal (3 papers on PubPeer for him), now Director Of Brain tumor Research at the University of Mississippi Medical Center in Jackson.
This fairy-tale in the shape of a scientific paper claims that α2-Macroglobulin and CS-GRP78 regulate acetyl-CoA synthesis and thus function as epigenetic modulators by enhancing histone acetylation in cancer cells. Look at this joke:
Udhayakumar Gopal, Salvatore V. Pizzo* Cell surface GRP78 promotes tumor cell histone acetylation through metabolic reprogramming: a mechanism which modulates the Warburg effect Oncotarget (2017) doi: 10.18632/oncotarget.22431



Another blot describes both P-FoxO1 and P-ACLY (magenta boxes) in three different cell types (A375, 1-LN, and A172), in one case it was mirrored horizontally.




Even more recent is the next work from 2019, published in the Journal of Biological Chemistry, a journal that used to be at the forefront against science fraud, whereas now is just one of the many Elsevier’s dustbins.
How JBC sold its Soul to the Devil
All good things come to an end.
The first author is again Gopal, while in second position we find Yvonne Mowery (3 papers on PubPeer, all with Sal), who moved in 2023 from Duke to the UPMC Hillman Cancer Center in Pittsburg as associate professor in radiation oncology. In third position in the author list sits a certain Kenneth Young, who is a lab administrator at DUMC in the department of radiation oncology. The paper investigates how GRP78 contributes to radio-resistance in pancreatic ductal adenocarcinoma (PDAC) cells. Remarkably, the authors found out that targeting GRP78 with C38 monoclonal antibodies increased the efficacy of radiation therapy against PDAC. Pity that all is fake:
Udhayakumar Gopal, Yvonne Mowery, Kenneth Young, Salvatore Vincent Pizzo* Targeting cell surface GRP78 enhances pancreatic cancer radiosensitivity through YAP/TAZ protein signaling Journal of Biological Chemistry (2019) doi: 10.1074/jbc.ra119.009091



Although it’s not one of the worst, the next JBC paper is worth a mention because is co-signed by Gopal again and by Mario Gonzalez-Gronow, associate professor emeritus of pathology at DUMC. The article allegedly demonstrates that α2M*/CS-GRP78 acts as an upstream regulator of the PDK1/PLK1 signaling axis to modulate c-MYC transcription and its target genes. Thus the authors suggest a therapeutic strategy for targeting c-MYC-associated malignant progression.
The same GAPDH control has been reused for different cell types and treatments:
Udhayakumar Gopal, Mario Gonzalez-Gronow, Salvatore Vincent Pizzo* Activated α2-Macroglobulin Regulates Transcriptional Activation of c-MYC Target Genes through Cell Surface GRP78 Protein Journal of Biological Chemistry (2016) doi: 10.1074/jbc.m115.708131

Another one for Big Mario:
Mario Gonzalez-Gronow, Steven J. Kaczowka, Sturgis Payne, Fang Wang, Govind Gawdi, Salvatore V. Pizzo* Plasminogen Structural Domains Exhibit Different Functions When Associated with Cell Surface GRP78 or the Voltage-dependent Anion Channel Journal of Biological Chemistry (2007) doi: 10.1074/jbc.m703342200

The legacy of Super Mario again:
Uma Kant Misra, Mario Gonzalez-Gronow, Govind Gawdi, Fang Wang, Salvatore Vincent Pizzo* A novel receptor function for the heat shock protein Grp78: silencing of Grp78 gene expression attenuates α2M*-induced signalling Cellular Signalling (2004) doi: 10.1016/j.cellsig.2004.01.003

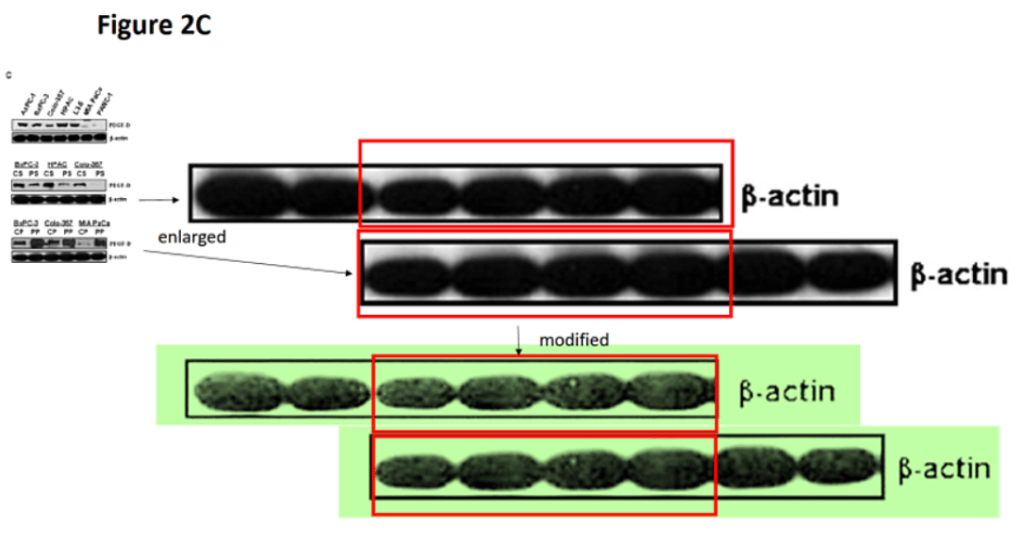
Yet the absolute masterpiece by Sal and Uma is the next one. The article was published in 2012 when they were aged 70 and 90, respectively. No-one at Duke wondered how these two guys were capable of producing so much data all alone. Six blots are reused within the paper, another ten are shared across eight other works, namely Mol Cancer Ther 2009, JBC 2011, J Cell Biochem 2011, PLoS One 2013, PLoS One 2014, JBC 2006, JBC 2005, J Leuk Biol 2005), each time describing different things.
Credits go to Clare Francis for digging out this mess. The article allegedly demonstrates that the binding of α2M* to prostate cancer cell surface GRP78 upregulates mTORC1 and mTORC2 activation and promotes protein synthesis in the prostate cancer cells. Unfortunately nothing in this paper can be taken seriously:
Uma K. Misra , Salvatore V. Pizzo* Receptor-Recognized α2-Macroglobulin Binds to Cell Surface-Associated GRP78 and Activates mTORC1 and mTORC2 Signaling in Prostate Cancer Cells PLoS ONE (2012) doi: 10.1371/journal.pone.0051735












Most of those issues illustrated above were identified using ImageTwin.
On PubPeer thirteen papers are co-signed by only Sal and Uma, while ten others have three authors with Uma first and Sal last, plus one middle author. Other recurrent names on Sal’s papers are Steven J. Kaczowka, Sturgis Payne, Govind Gawdi and Rohit Deedwania.
One thing is certain: anyone in Carolina with issues of prostate cancer better DON’T call Sal.
Look at this stuff:
Uma K. Misra, Sturgis Payne, Salvatore V. Pizzo* The Monomeric Receptor Binding Domain of Tetrameric α2-Macroglobulin Binds to Cell Surface GRP78 Triggering Equivalent Activation of Signaling Cascades Biochemistry (2013) doi: 10.1021/bi400376s



The most funny one:

Uma Kant Misra, Govind Gawdi, Salvatore Vincent Pizzo* Induction of mitogenic signalling in the 1LN prostate cell line on exposure to submicromolar concentrations of cadmium+ Cellular Signalling (2003) doi: 10.1016/s0898-6568(03)00117-7

Eye rolling? Right. I’ll stop here showing Sal & Co’s scientific atrocities, I believe the message is pretty clear.
Leonid wrote to Sal and to several Duke University’s executives for explanations, including Duke’s research integrity officer Dr Donna Kessler.
Sal’s initial attitude of denial first and threatening later, together with the worrisome “no reply” strategy by Dr Kessler, was abandoned due to Leonid’s persistence and the piling-up of issues on PubPeer. In fact, suddenly the following email materialized. It is from Alan D. Proia, who holds a double professorship in pathology and ophthalmology (highlights mine):
“Dear Dr. Schneider,
Dr. Pizzo requested that I independently review the images in question since I did not participate in the research and have no vested interest in the publications.
I agree that the images you questioned appear to have been duplicated with different labels in the publications. I base this opinion on 44 years in pathology, which requires comparing images as part of my routine practice. I also have a Ph.D. in biochemistry so I am proficient in comparing gel images.
Dr. Pizzo is extremely concerned and upset that errors, of which he was unaware, may have occurred. Dr. Pizzo has authorized me to request a complete review of this issue from Dr. Donna Kessler’s scientific misconduct review office of Duke University.
Dr. Pizzo believes that all the notebooks with original data are still in storage, which will facilitate an investigation. He has also requested that Dr. Kristi Orstian assist Dr. Kessler if help is needed with the review.
Dr. Pizzo thanks you for bringing this matter to his attention. He is committed to an open and comprehensive investigation of this matter and hopes it can be conducted swiftly.
Please let me know if you have any other concerns that I may help address.
Sincerely yours,
Alan D. Proia, M.D., Ph.D.”
In the string of attached emails indeed Sal asked to Dr Proia to take care of this can of worms:
“Alan, I need advice. Could you call sometime. Sal“
Yes Alan, you better call Sal. However, in all honesty, I wonder why it is Sal himself deciding who should review his own papers. Second, since it is Dr Kessler the head of the scientific misconduct review office of Duke, then what is she exactly paid for?
Perhaps I’m nitpicking. After all an internal investigation is ongoing now, no?
The Name of the Foes
“I am Jorge de Burgos. I believe research should pause in searching for the progress of knowledge. Right now, we don’t need more papers, we rather need more knowledge by going through a continuous and sublime recapitulation to figure out what is true and what is fake” – Aneurus Inconstans
Dr Proia has also promptly contacted Richard C. Austin, professor for cardiovascular and kidney disease at the McMaster University and St. Joseph’s Healthcare Hamilton, Hamilton, Ontario. Austin is the corresponding author of one of the works where Sal is just a middle author. I’m truly impressed.
The paper by the Austin Lab, where Sal is a co-author, allegedly demonstrates that heparin derivatives abrogate the anti-GRP78 autoantibody induction of tumor cell proliferation (which occurs through a TF-dependent mechanism) by blocking AutoAb binding to the cell-surface of cancer cells from prostate cancer patients.
Here is the issue in that paper, one blot describes different proteins:

Ali A. Al-Hashimi, Paul Lebeau, Fadwa Majeed, Enio Polena, Šárka Lhotak , Celeste A. F. Collins, Jehonathan H. Pinthus, Mario Gonzalez-Gronow, Jen Hoogenes, Salvatore V. Pizzo, Mark Crowther, Anil Kapoor, Janusz Rak, Gabriel Gyulay, Sara D’Angelo, Serena Marchiò, Renata Pasqualini, Wadih Arap, Bobby Shayegan, Richard C. Austin* Autoantibodies against the cell surface-associated chaperone GRP78 stimulate tumor growth via tissue factor Journal of Biological Chemistry (2017) doi: 10.1074/jbc.m117.799908
You can appreciate this is not a simple duplication as the TF and p-eIF2α blots are cropped differently. Dr Austin offered his thoughts via email on the matter (highlights mine):
“Dear Everyone,
Richard C. Austin
the image in question was generated by the first author of the study, Dr. Ali Al-Hashimi. I believe that this was an error during the submission of the original images involved. I have asked Dr. Al-Hashimi to provide the original blot for the p-eIF2alpha and submit the correct figure. I want to emphasize that this error in the image has no bearing on the findings of the paper as we examined multiple markers of ER stress, both at the mRNA and protein level. I will respond once Dr. Al-Hashimi determined where the error occurred and to provide the correct image. “
Asking the first author to provide the original blot for the p-eIF2α would mean Austin found out the erroneous blot was that one and not TF? But then why waiting for Dr. Al-Hashimi to determine where the error occurred?
The Fellow of Canadian Academy of Health Sciences (appointed in 2022) says that the error has no bearing on whatsoever. How does Dr Austin know when he is still investigating?
Unfortunately, this is not the only error in Dr Austin’s papers, 9 of his articles are flagged on PubPeer for pretty bad stuff. Here is one, posted in September 2022 by our sleuth colleague Cheshire, two sets of micrographs overlap and are supposed to describe different genotypes and treatments:
Byun JH, Lebeau PF, Platko K, Carlisle RE, Faiyaz M, Chen J, MacDonald ME, Makda Y, Yousof T, Lynn EG, Dickhout JG, Krepinsky JC, Weaver F, Igdoura SA, Seidah NG, Austin RC* Inhibitory Antibodies against PCSK9 Reduce Surface CD36 and Mitigate Diet-Induced Renal Lipotoxicity Kidney360 (2022) doi: 10.34067/kid.0007022021
On PubPeer Dr Austin was again keen to reassure there’s no bearing:
“Thank for pointing out this error in these images. This happened during the processing of the manuscript and has no bearing on the final results of the study. We have the original images that were incorrectly left out of the final image and will let the journal know about this error. There are no other errors within the paper and we thank PuPeer for pointing this out. Sincerely, Rick Austin”
Somehow Austin and his people cannot place two micrographs together without doing a mess. How is that?
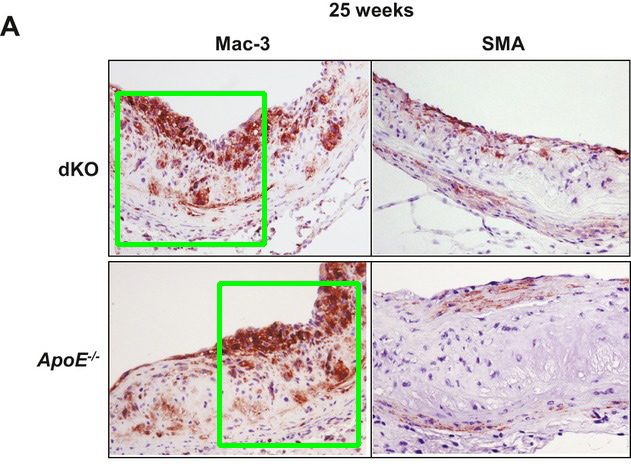
Hossain GS, Lynn EG, Maclean KN, Zhou J, Dickhout JG, Lhoták S, Trigatti B, Capone J, Rho J, Tang D, McCulloch CA, Al-Bondokji I, Malloy MJ, Pullinger CR, Kane JP, Li Y, Shiffman D, Austin RC* Deficiency of TDAG51 protects against atherosclerosis by modulating apoptosis, cholesterol efflux, and peroxiredoxin-1 expression J Am Heart Assoc. (2013) doi: 10.1161/jaha.113.000134

Werstuck GH, Lentz SR, Dayal S, Hossain GS, Sood SK, Shi YY, Zhou J, Maeda N, Krisans SK, Malinow MR, Austin RC* Homocysteine-induced endoplasmic reticulum stress causes dysregulation of the cholesterol and triglyceride biosynthetic pathways J Clinical Invest. (2001) doi: 10.1172/jci11596
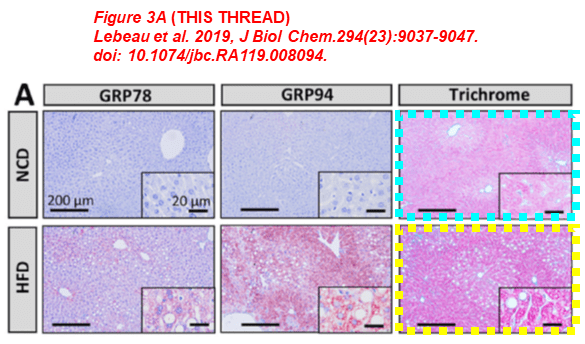
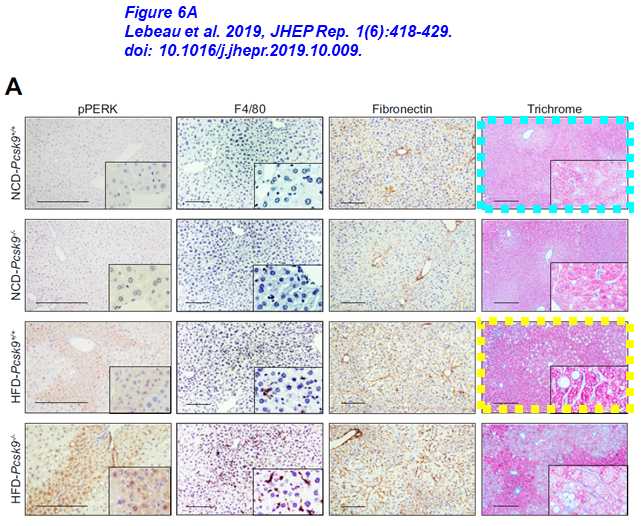
Below another example by Austin. Micrographs (blue and green boxes) have been reused across articles for different genotypes (Pcsk9-/- and Pcsk9+/+, respectively). On top of that, another four sets of images (dashed boxes) were reused without any disclosure in the two papers published shortly apart. Do they take one single image for each experiment, which in turn is never repeated a second time?
Lebeau PF, Byun JH, Platko K, MacDonald ME, Poon SV, Faiyaz M, Seidah NG, Austin RC* Diet-induced hepatic steatosis abrogates cell-surface LDLR by inducing PCSK9 expression in mice J Biol Chem. (2019) doi: 10.1074/jbc.ra119.008094
Lebeau PF, Byun JH, Platko K, Al-Hashimi AA, Lhoták Š, MacDonald ME, Mejia-Benitez A, Prat A, Igdoura SA, Trigatti B, Maclean KN, Seidah NG, Austin RC* Pcsk9 knockout exacerbates diet-induced non-alcoholic steatohepatitis, fibrosis and liver injury in mice JHEP Reports (2019) doi: 10.1016/j.jhepr.2019.10.009



But as seen before, it doesn’t go any better with Western blots for Austin. In the paper below the same control is used for different treatments. It’s a collaborative work with Austin’s department colleague Geoff H. Werstuck, who is incidentally also the first author of one of the papers highlighted above:
Anna J. Kim , Yuanyuan Shi , Richard C. Austin, Geoff H. Werstuck* Valproate protects cells from ER stress-induced lipid accumulation and apoptosis by inhibiting glycogen synthase kinase-3 Journal of Cell Science (2005) doi: 10.1242/jcs.01562

Do you now understand, dear readers, in what hands cancer research is?

_____
More luminaries at Duke and around the USA
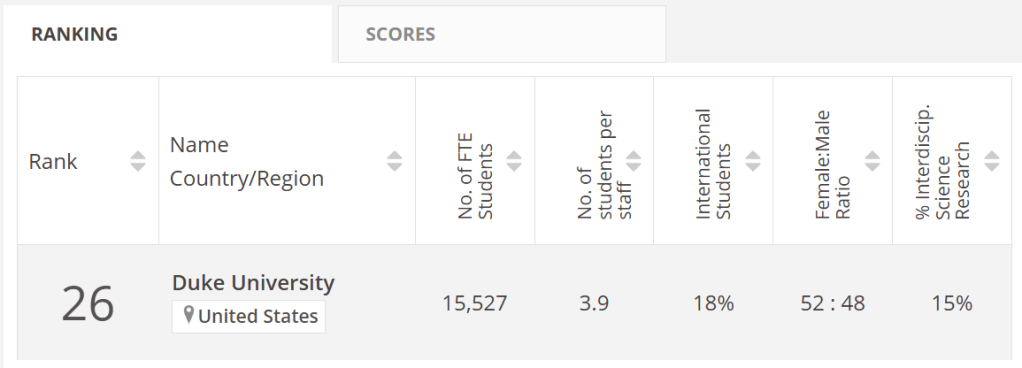
We leave the wilderness of Ontario and return to Duke. According to the Times Higher Education (THE) university ranking, Duke is 26th in the world, while it’s 57th in the QS ranking.
It should then be truly an amazing institution where the eager young minds of tomorrow are nurtured, no?
I wandered through the faculty members of DUMC and, almost instantaneously, the third and the fourth and the fifth person I checked fell into the net of my scrutiny. Can you believe it?
Please meet James L. Abbruzzese (15 papers on PubPeer, 5 retractions), distinguished professor emeritus of medical oncology, specialized in clinical study and treatment of pancreatic cancer. To be fair, Dr Abbruzzese is always a middle author on those articles. In fact, most of Abbruzzese’s problematic papers have as leading authors these two guys:
- Paul J. Chiao (25 papers on PubPeer, 1 retraction and 1 EoC), professor at MD Anderson Cancer Center in Houston, Texas (a place that For Better Science readers know like a second home)
- Fazlul H. Sarkar, sacked professor of pathology at the Karmanos Cancer Center of the Wayne State University in Detroit, Michigan, who sports 79 articles on PubPeer with 41 retractions (FORTY-ONE)
Important to say, while Sarkar was sacked and Abbruzzese has retired, Chiao is still out there doing damage. But what else could one expect from MD Anderson other than protecting its fleet of crooks?
Anil Sood and how much MD Anderson doesn’t care: whistleblowers speak out
“The graduate school at University of Texas MD Anderson does not care and keep sending students to his lab, Sood is a member of faculty there. RIO at MDACC doesn’t care because witnesses either left the country or are too afraid to speak.”
Below is one of Chiao’s & Abbruzzese’s co-productions. It’s pure beauty. Please pay attention to the background’s smoothness behind the copy-pasted bands:
Zhongkui Li, Guido M. Sclabas, Bailu Peng, Kenneth R. Hess, James L. Abbruzzese, Douglas B. Evans, Paul J. Chiao* Overexpression of synuclein-gamma in pancreatic adenocarcinoma Cancer (2004) doi: 10.1002/cncr.20321

Again the magic Chiao-Abbruzzese duo, everything is beautifully fake:
Fujioka S, Schmidt C, Sclabas GM, Li Z, Pelicano H, Peng B, Yao A, Niu J, Zhang W, Evans DB, Abbruzzese JL, Huang P, Chiao PJ* Stabilization of p53 is a novel mechanism for proapoptotic function of NF-kappaB J Biol Chem (2004) doi: 10.1074/jbc.m313435200



One of Abbruzzese’s retractions with Sarkar:
Zhiwei Wang, Dejuan Kong, Sanjeev Banerjee, Yiwei Li, N. Volkan Adsay, James Abbruzzese, Fazlul H. Sarkar* Down-regulation of platelet-derived growth factor-D inhibits cell growth and angiogenesis through inactivation of Notch-1 and nuclear factor-kappaB signaling Cancer Research (2007) doi: 10.1158/0008-5472.can-07-2803 [Retracted]
And then, icing on the cake. Below is a retracted paper bearing Abbruzzese, Chiao and Sarkar all together. The same actin control is duplicated, flipped and rotated three times, plus there are other duplications that were never illustrated on PubPeer:
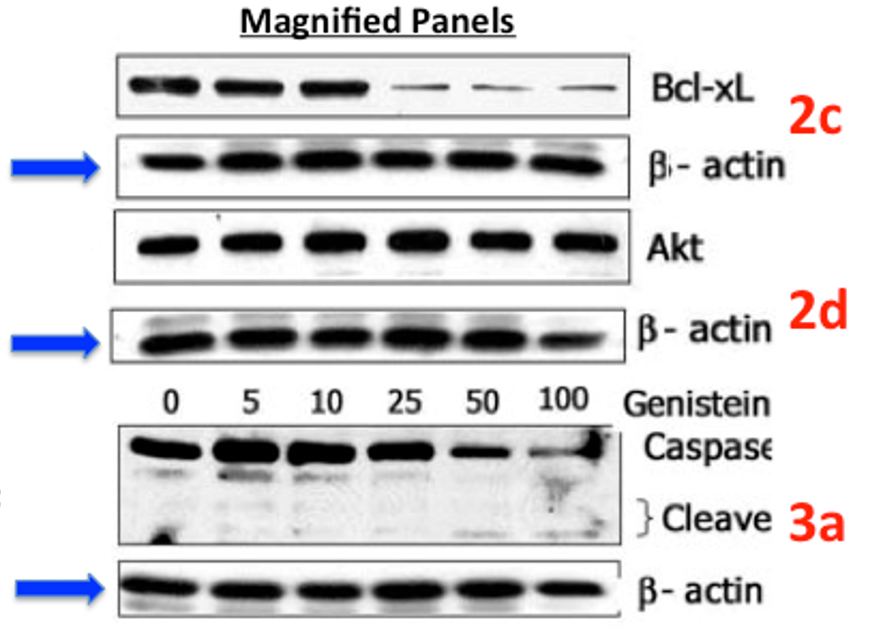
Sanjeev Banerjee, Yuxiang Zhang, Zhiwei Wang, Mingxin Che, Paul J. Chiao, James L Abbruzzese, Fazlul H. Sarkar* In vitro and in vivo molecular evidence of genistein action in augmenting the efficacy of cisplatin in pancreatic cancer Int J Cancer (2007) doi: 10.1002/ijc.22332 [Retracted]
Both Chiao and Sarkar would deserve their own FBS article, but we have no time and have to move one.
A few fake papers also for Darell Doty Bigner, E. L. and Lucille F. Jones Cancer Distinguished Research Professor in the School of Medicine at DUMC, a guy bearing also the titles of Professor of Neurosurgery, Surgery and Pathology. A quadruple professorship, and 3 articles tagged for him, although he is always a middle author. For instance the JBC paper below is from 2013 and was corrected in 2018. I made a post-correction assessment on PubPeer a few weeks ago:
Poteet E, Choudhury GR, Winters A, Li W, Ryou MG, Liu R, Tang L, Ghorpade A, Wen Y, Yuan F, Keir ST, Yan H, Bigner DD, Simpkins JW, Yang SH* Reversing the Warburg Effect as a Treatment for Glioblastoma J Biol Chem. (2013) doi: 10.1074/jbc.M112.440354.


The corresponding author Shao-Hua Yang, 3 papers on PubPeer professor of pharmacology and neuroscience at the Institute for Healthy Aging of the University of North Texas Health Science Center at Fort Worth, felt the necessity to clarify a few things on PubPeer (highlights mine):
“The justification has been provided to the editor in the correction response in 2018. We have provided editor the original whole blot membranes images, invert images, and images with brightness adjustment. The p-mTOR and mTOR in 5B, although look very similar in the original figure, they are very different after brightness adjustment.”
S.-H. Yang
You tell me, dear readers, if p-mTOR and mTOR can be different:
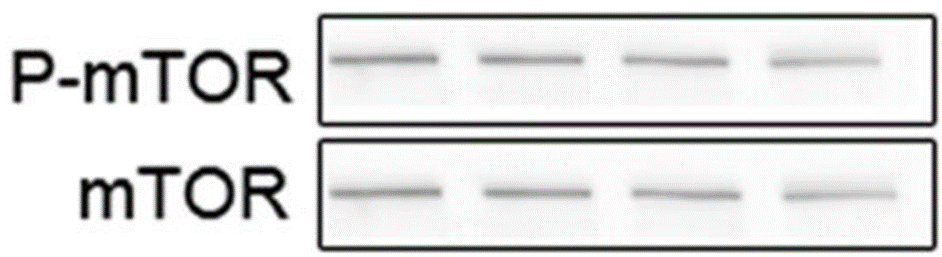
Again Dr Yang wanted to dispel any doubts:
“The ACC in 6C and Cyclin E1 in 6D, although look very similar in the original figure, they had very different molecular weight. The ACC blots were at the top section of the membrane, while the Cyclin E1 were at the middle section of the membrane. To avoid future confusion, we have provided the editor additional immunoblots (look very different) for the correction.”
S.-H. Yang
They had very different molecular weight? Of course dear Dr Yang!
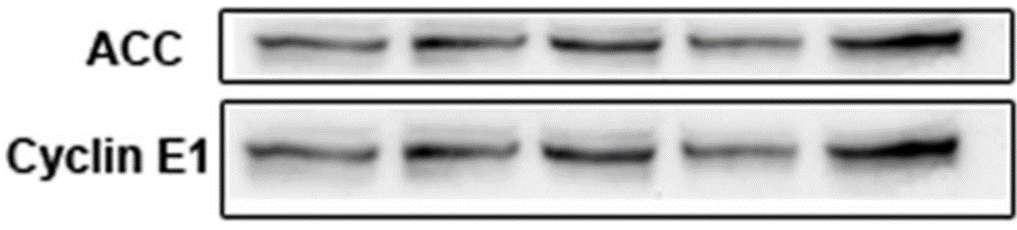
I’m afraid that Dr Yang torpedoed himself. Either he is visually impaired or he is cheating, and I’ll leave you to imagine which option I’m leaning towards, dear readers.
Below is another gem by the four-time-professor Bigner, this time in collaboration with Shi-Yuan Cheng, almost 30 papers on PubPeer, professor of neurology and neuro-oncology at the Feinberg School of Medicine of the Northwestern University in Chicago, Illinois. The paper was dissected by our sleuth colleague Cheshire, who scrutinized even the supplementary figures. Please look carefully to understand in what hands cancer research truly is:
Feng H, Lopez GY, Kim CK, Alvarez A, Duncan CG, Nishikawa R, Nagane M, Su AJ, Auron PE, Hedberg ML, Wang L, Raizer JJ, Kessler JA, Parsa AT, Gao WQ, Kim SH, Minata M, Nakano I, Grandis JR, McLendon RE, Bigner DD, Lin HK, Furnari FB, Cavenee WK, Hu B, Yan H, Cheng SY* EGFR phosphorylation of DCBLD2 recruits TRAF6 and stimulates AKT-promoted tumorigenesis J Clin Invest (2014) doi: 10.1172/jci73093

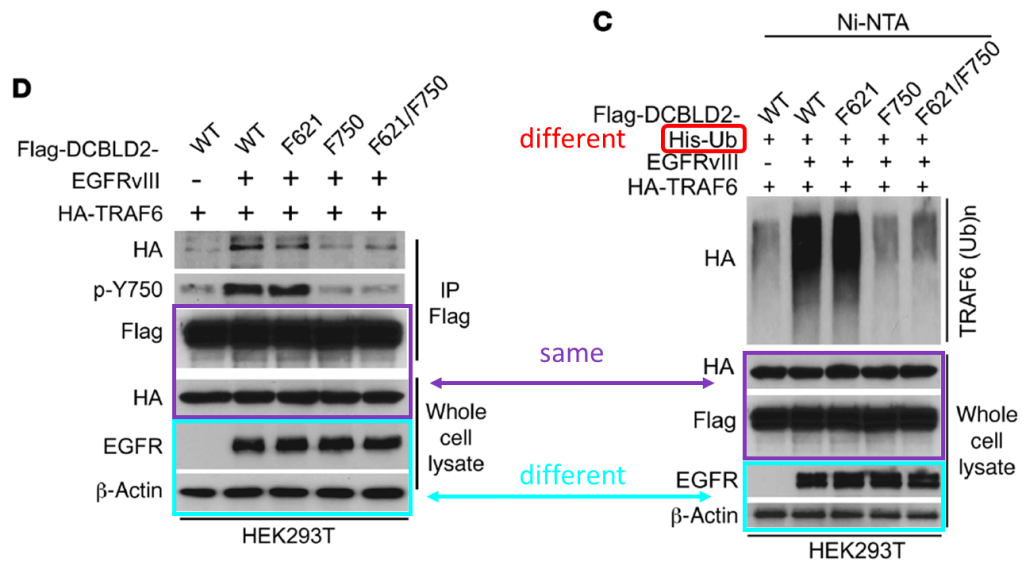
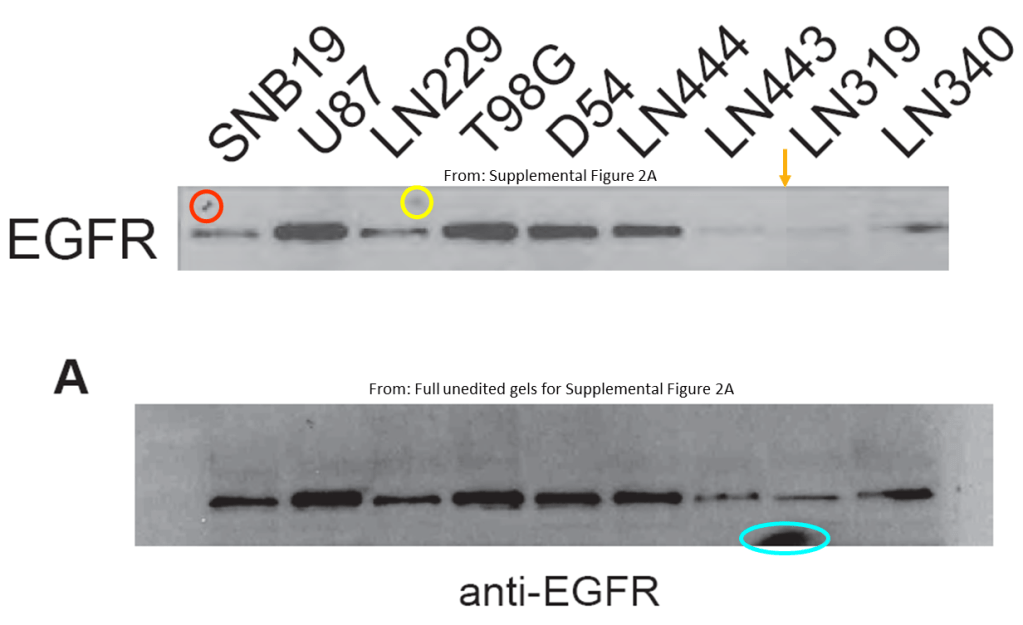
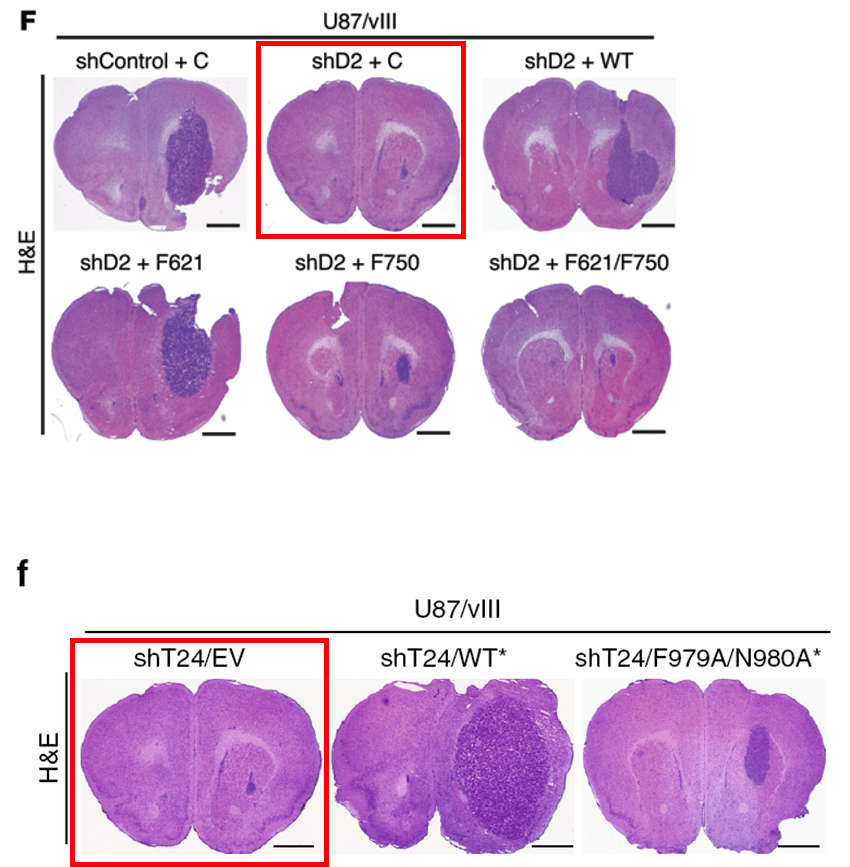
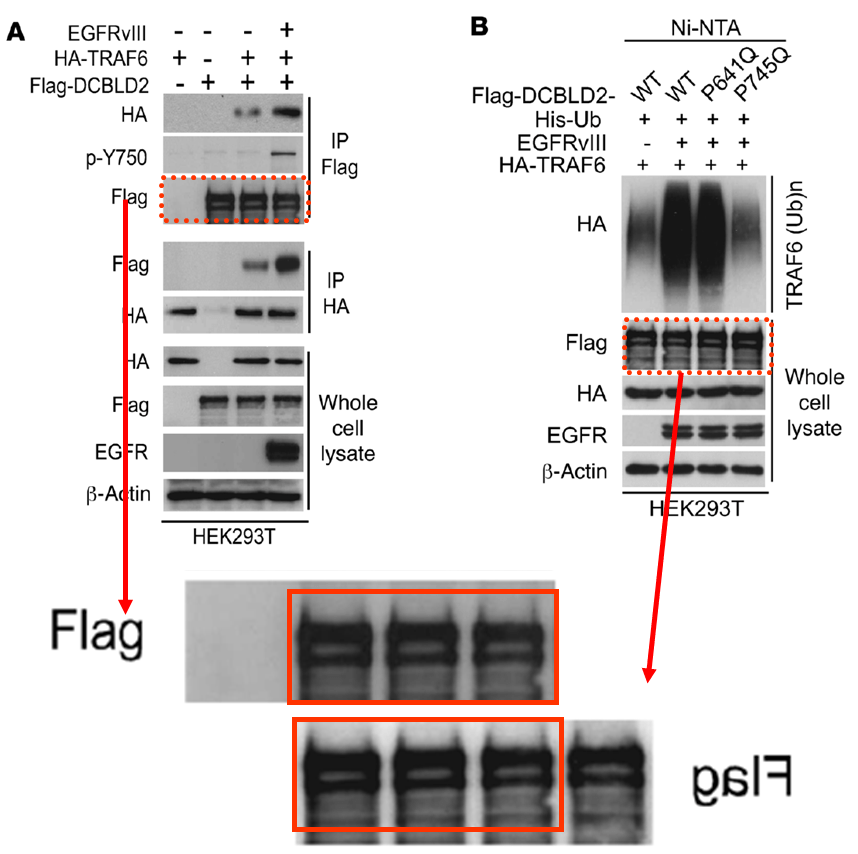
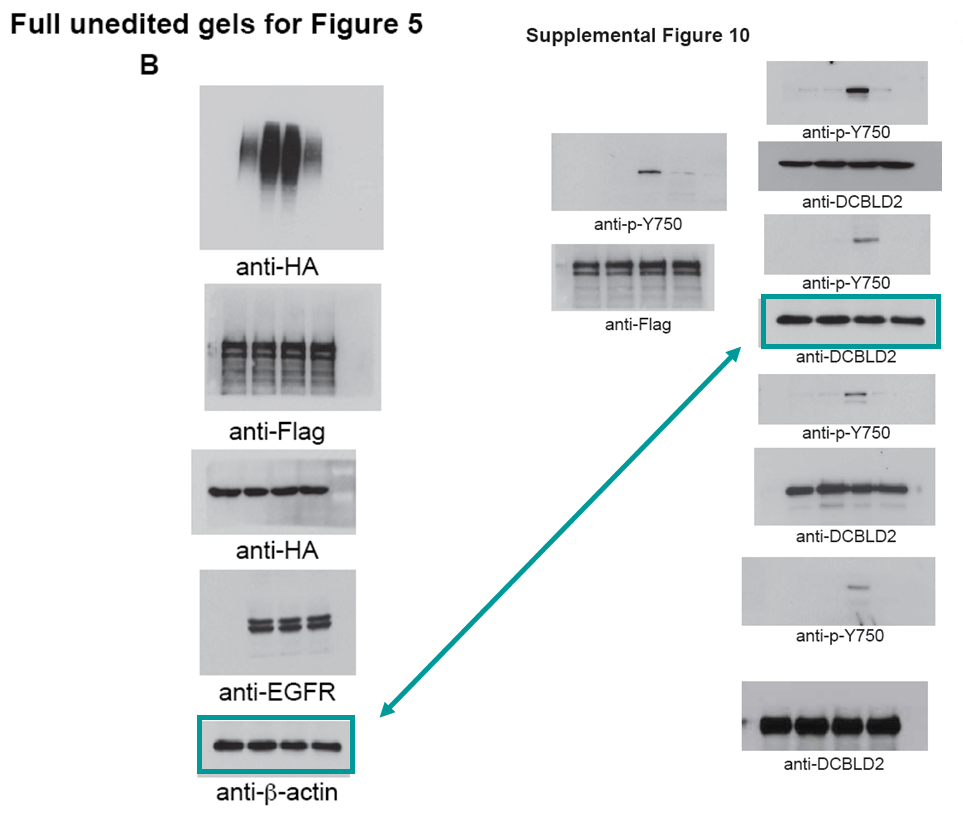
Below is another Cheng’s work that aims to defeat cancer, and once more Cheshire’s grin floated in. You’ll find again the whole package: duplicated and rotated micrographs and blots within and across papers describing different things:
Haizhong Feng , Bo Hu , Kun-Wei Liu , Yanxin Li , Xinghua Lu , Tao Cheng , Jia-Jean Yiin , Songjian Lu , Susan Keezer , Tim Fenton , Frank B. Furnari , Ronald L. Hamilton , Kristiina Vuori , Jann N. Sarkaria , Motoo Nagane , Ryo Nishikawa , Webster K. Cavenee , Shi-Yuan Cheng* Activation of Rac1 by Src-dependent phosphorylation of Dock180Y1811 mediates PDGFRα-stimulated glioma tumorigenesis in mice and humans J Clin Invest (2011) doi: 10.1172/jci58559



Those highlighted numbers in the caption above (listed by Cheshire) are the grants spent (or partly spent) to produce that fairy-tale. This is what your money is used for, dear taxpayers.
Perhaps you can spot some recurring co-authors in Cheng’s fake papers. Please meet Haizhong Feng (10 papers on PubPeer) currently professor at the Renji Hospital of the Shanghai Jiao Tong University, Frank B. Furnari (11 papers on PubPeer) professor of medicine at the Moores Cancer Center of UC San Diego, and Webster K. Cavenee (11 papers on PubPeer) distinguished professor at the Ludwig Institute for Cancer Research of UCSD.
Here below another lovely Cheng-Feng-Cavenee-Furnari production, this time in PNAS no the less, “Contributed by Webster K. Cavenee“, the National Academy of Sciences member:
Haizhong Feng, Bo Hu, Michael J Jarzynka, Yanxin Li, Susan Keezer, Terrance G Johns, Careen K Tang, Ronald L Hamilton, Kristiina Vuori, Ryo Nishikawa, Jann N Sarkaria, Tim Fenton, Tao Cheng, Frank B Furnari, Webster K Cavenee, Shi-Yuan Cheng* Phosphorylation of dedicator of cytokinesis 1 (Dock180) at tyrosine residue Y722 by Src family kinases mediates EGFRvIII-driven glioblastoma tumorigenesis PNAS (2012) doi: 10.1073/pnas.1121457109

Back to Duke.
Wandering around Duke’s faculty corridors brought me to Ming Chen, associate professor in pathology at DUMC, who sports just 3 papers on PubPeer, but all three include very problematic names. Below is a worrisome Nature Genetics paper flagged by our sleuth colleague Sholto David in January 2024, where Dr Chen is the first author:
Chen M, Zhang J, Sampieri K, Clohessy JG, Mendez L, Gonzalez-Billalabeitia E, Liu XS, Lee YR, Fung J, Katon JM, Menon AV, Webster KA, Ng C, Palumbieri MD, Diolombi MS, Breitkopf SB, Teruya-Feldstein J, Signoretti S, Bronson RT, Asara JM, Castillo-Martin M, Cordon-Cardo C, Pandolfi PP* An aberrant SREBP-dependent lipogenic program promotes metastatic prostate cancer Nature Genetics (2018) doi: 10.1038/s41588-017-0027-2.

The constellation of authors in the paper described above deserves a presentation.

Pier Paolo Pandolfi out of Harvard, spotted in Italy and Nevada
Star cancer researcher Pier Paolo Pandolfi left Harvard. The allegations are very serious, but do his new employers in Nevada and Italy mind?
- The corresponding author is Pier Paolo Pandolfi (43 papers on PubPeer), sacked Harvard professor, now scientific director at the William N. Pennington Cancer Institute in Reno, Nevada. Chen and Pandolfi used to work together at the Beth Israel Deaconess Cancer Center of Harvard Medical School before Pandolfi was kicked out for sexual harassment. All three Chen’s paper on PubPeer are with Pandolfi.
- Second-last name is Carlos Cordon-Cardo (33 papers on PubPeer), chair of the Department of Pathology, Molecular and Cell-Based Medicine at the Icahn School of Medicine Mount Sinai, New York City.
- Third-last name is Mireia Castillo-Martin (8 papers on PubPeer), previously also at Mount Sinai, now research group leader at the Champalimaud Foundation in Lisbon, Portugal.
- Fourth-last is John M. Asara (16 papers on PubPeer), associate professor and director of the mass spectrometry core facility at Beth Israel Deaconess Cancer Center of Harvard Medical School.
- Fifth-last is Roderick T. Bronson (14 papers on PubPeer) core unit director at the Department of Microbiology and Immunobiology, Harvard Medical School.
- Sixth-last is Sabina Signoretti (25 papers on PubPeer), researcher at Dana-Farber Cancer Institute in Boston, Massachusetts, another place infested by an incredible number of questionable scientists.
- Seventh-last is Julie Teruya-Feldstein (8 papers on PubPeer), director of hematopathology at the Icahn School of Medicine Mount Sinai, NYC.
Dana-Farberications at Harvard University
“Imagine what mistakes might be found in the raw data if anyone was allowed to look!” – Sholto David
There’s no time now to go through the questionable science by those names listed above. Just know that if you click on the links to their pubpeered articles you will open a whole new Pandora’s box and arrive at Lewis Cantley, Ronald DePinho, Douglas Green, John Blenis, Wenyi Wei, George Daley, Raghu Kalluri, Giulio Draetta, James Collins and hundreds more. Please check it out yourselves if you care to know how deep the rabbit hole goes.
Does anyone know if the tragic characters I discussed in this blog post also have a role as hospital doctors? That is, in addition to producing bogus data, do they also treat patients? While waiting to discover the mystery, I extend my heartfelt wishes of a speedy recovery to all cancer patients all over the globe, and today in particular to those of Carolina. Yes, I’m gone to Carolina in my mind.
Donate!
If you are interested to support my work, you can leave here a small tip of $5. Or several of small tips, just increase the amount as you like (2x=€10; 5x=€25). Your generous patronage of my journalism will be most appreciated!
€5.00




















A very nice and comprehensive piece, I won’t tire of these. Some familiar faces, but mostly brand-new ones. The thought of these old men pottering around in the lab doing western blots is charming, so it’s a shame that never happened. Sabina Signoretti is one of my all-time favourites, a frequent collaborator of another top Italian scientist – Massimo Loda – he’s always worth keeping in the back of your mind.
LikeLiked by 2 people
The are numerous clear data manipulation in the work of Pizzo, e.g.,
https://pubpeer.com/publications/8412E0B4FC46990FA432C28DE38A85
Instead of attacking the data sleuths he may fix these records.
LikeLiked by 1 person
Duke University seems to fester cancer quacks, dating back at least to the infamy of Anil Potti. What’s most shocking is that cancer patients were given therapies based on phony data. Sal’s attempt at intimidation “…You give me the name of your lawyer…” is laughable.
LikeLiked by 2 people
Yes, the Potti-Nevins case was terrible… and minimized by the Duke vice deans who did everything to cover up the fraud, Michael Cuffe for medical affairs and – guess who – Sally Kornbluth for research, currently president of the MIT. I tell the story (sorry, in French) in doi: 10.1016/j.bulcan.2020.12.004
LikeLiked by 1 person
“Uma Kant Misra, Salvatore Vincent Pizzo* Up-regulation of GRP78 and antiapoptotic signaling in murine peritoneal macrophages exposed to insulin Journal of Leukocyte Biology (2005) doi: 10.1189/jlb.1104685“
Stock in trade for that journal.
See: PubPeer – NF-kappaB-dependent control of HIV-1 transcription by the se…
Science and the Journal of Leukocyte Biology go together like oil and water! The journal is steadfast about doing nothing whatsoever about the problematic data.
LikeLiked by 2 people
The constant emphasis on ‘errors’ is becoming quite exhausting. How can there be so many “errors”across so many figures in so many peer-reviewed papers?!
LikeLike
The error was to have been caught.
LikeLike
One more for Rick “No Bearing” Austin, 10 for him on PubPeer:
https://pubpeer.com/publications/96FEB6257F86BD561230952E2DF438#1
LikeLike
More issues for James Abbruzzese, flagged by Clare Francis minutes ago:
PubPeer – Bortezomib inhibits PKR-like endoplasmic reticulum (ER) kina…
This one too:
PubPeer – Effects of the proteasome inhibitor PS-341 on apoptosis and…
The corresponding author of both papers is David J. McConkey, professor at Johns Hopkins University, please read his CV and where he has worked:
David McConkey – Johns Hopkins Physical Sciences Oncology Center (jhu.edu)
LikeLike
24 October 2024 Expression of Concern for James L. Abbruzzese and Paul Chiao.
https://acsjournals.onlinelibrary.wiley.com/doi/10.1002/cncr.35605
LikeLiked by 1 person
Editor’s note for James L. Abbruzzese and Paul Chiao.
https://aacrjournals.org/clincancerres/article/30/23/5495/750206/Editor-s-Note-Deciphering-the-Mechanisms-of
The images have been deciphered. They are the same.
LikeLike
09 December 2024 Editor’s Note for James L. Abbruzzese and Paul J. Chiao
09 December 2024 Editor’s Note.
https://academic.oup.com/jnci/advance-article/doi/10.1093/jnci/djae312/7919713?login=false
This is an Editor’s Note regarding: Davide Melisi, Qianghua Xia, Genni Paradiso, Jianhua Ling, Tania Moccia, Carmine Carbone, Alfredo Budillon,
, Modulation of Pancreatic Cancer Chemoresistance by Inhibition of TAK1, J Natl Cancer Inst, Volume 103, Issue 15, 3 August 2011, Pages 1190–1204, https://doi.org/10.1093/jnci/djr243
In September 2024, a reader contacted the journal about similarities in the bands for beta-actin in Figure 1B and I. They also referred to a post on PubPeer (https://pubpeer.com/publications/CF2ADDEEA370F6E995F4D46ADB7B27). The Editors contacted the authors, who admitted the image in Figure 1B was reused in Figure 1I. They explained that the experimental conditions evaluated in 1B and I were identical. These figures represent the expression of TAK1 (Figure 1B) and cIAP-2, cleaved Caspase 3, cleaved PARP1, and TAK1 (Figure 1I) in protein samples extracted from the same cells under identical TAK1 expression conditions. Consequently, the beta-actin loading control image used in both panels was derived from the same blot, as these were not separate biological replicates. When the research was conducted, they deemed it unnecessary to run a separate blot for the beta-actin loading control.
The Editors disagree that a separate blot for beta-actin was unnecessary and advise readers to be cautious when interpreting these results.
All authors were notified that this Editor’s Note was being published but the journal could not find contact information for Genni Paradiso.
LikeLike
I wanted to say that the man that is coming after Sal : is being slanderous ! This article is misinformation and grounds for slander !!!!!!! I know all about his work and personally . This article really upset and disturbed me !
LikeLike
If it helps, try not to read this article too often.
LikeLike
“slanderous”. Isn’t slander about speech?
LikeLike
Just an EDITORIAL NOTE to Li et al. 2014 PLoS One, issued on 03 April 2025:
PubPeer – Model of tumor dormancy/recurrence after short-term chemothe…
Editorial Note: Model of tumor dormancy/recurrence after short-term chemotherapy | PLOS One
This is is one of Sal’s collaborations with Robin E. Bachelder. This time PLoS didn’t do a great job.
LikeLike
PLos One is re-calling Sal!
Retraction: Activated α2-macroglobulin binding to cell surface GRP78 induces T-loop phosphorylation of Akt1 by PDK1 in association with raptor | PLOS One
LikeLiked by 1 person
Another re-call for Sal! Retraction: Evidence for a Pro-proliferative Feedback Loop in Prostate Cancer: The role of Epac1 and COX-2-dependent Pathways | PLOS One
LikeLiked by 1 person
The third bus. Retraction: Receptor-Recognized α2-Macroglobulin Binds to Cell Surface-Associated GRP78 and Activates mTORC1 and mTORC2 Signaling in Prostate Cancer Cells – PubMed
LikeLiked by 1 person
Four more RETRACTIONS for Sal, all of them by the Journal of Cellular Biochemistry, 15 July 2025:
Journal of Cellular Biochemistry | Wiley Online Library
Journal of Cellular Biochemistry | Wiley Online Library
Journal of Cellular Biochemistry | Wiley Online Library
Journal of Cellular Biochemistry | Wiley Online Library
Now Sal stands at 7 retractions in total. We wish many more to come.
LikeLiked by 1 person
Total retractions appear to be 8.
Duke scientists lose eight papers for alleged image manipulation – Retraction Watch
LikeLike
https://retractionwatch.com/2025/09/29/duke-scientists-lose-eight-papers-for-alleged-image-manipulation/
LikeLike
Here is RETRACTION #8 for Sal, which escaped my notice on July 16, 2025:
Journal of Cellular Biochemistry | Wiley Online Library
Retraction Watch wrote a piece on this story yesterday Sept 29, 2025:
Duke scientists lose eight papers for alleged image manipulation – Retraction Watch
We learn that Uma Kant Misra passed away just few weeks ago:
Obituary information for Dr. Uma Kant Misra, Ph.D.
LikeLike
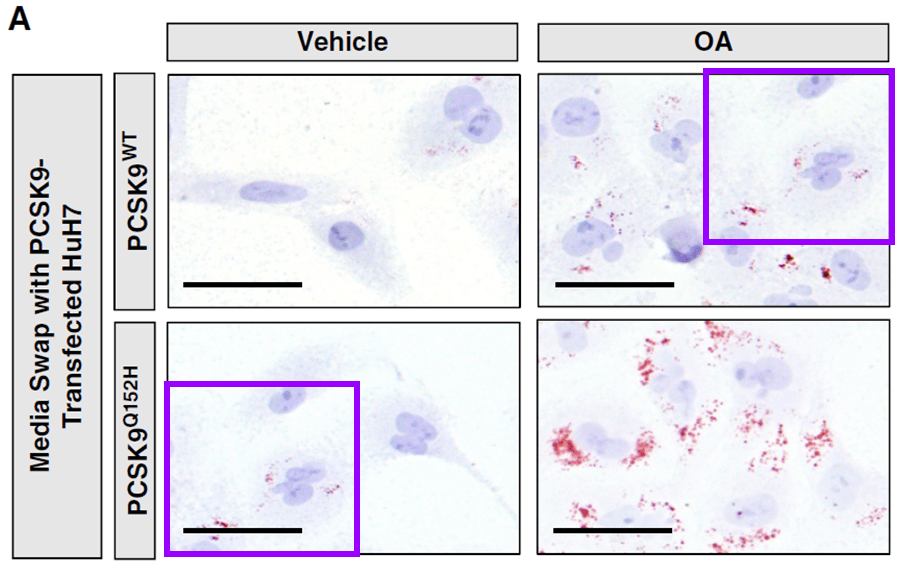
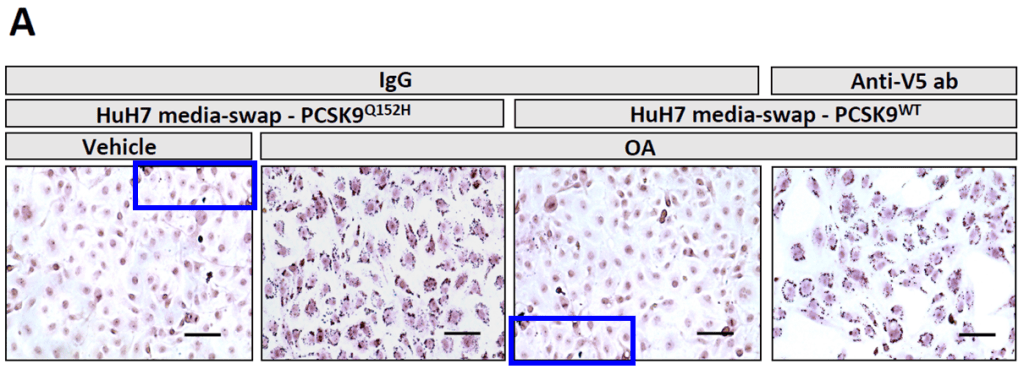
Qustionable CORRECTION to Al-Hashimi et al. 2017 J Biol Chem, 14 Aug 2025:
https://www.jbc.org/article/S0021-9258(25)02357-9/fulltext
https://pubpeer.com/publications/0F554B76849DBB67B4D0DE472D70A2
LikeLiked by 1 person