Smut Clyde is once again concerned about the state of Indian science. This times, it seems to have overdosed on taurine. Or maybe again on Ayurveda. In any case, it’s about antioxidants! Meet Professor Parames Chandra Sil of Bose Institute (curcumin nanoparticles as main research highlight!), and just when you have enough and would like to escape, Smut will make you meet another researcher from Kolkata: Professor Saikat Dewanjee of Jadavpur University (whom you had to sustain in last Friday Shorts).
Here is Sil’s PubPeer record of over 60 fraudulent papers, and here is Dewanjee’s, with over 30 fraudulent papers. Yes, there is also a spreadsheet, with a sub-spreadsheet!

Bosone Layer
By Smut Clyde
I cannot turn my eyes away from the central panel here. When PubPeer contributors die and go to Hell for being PubPeer contributors, it is how their private rooms are wallpapered. This travesty earned 98 citations for its authors.
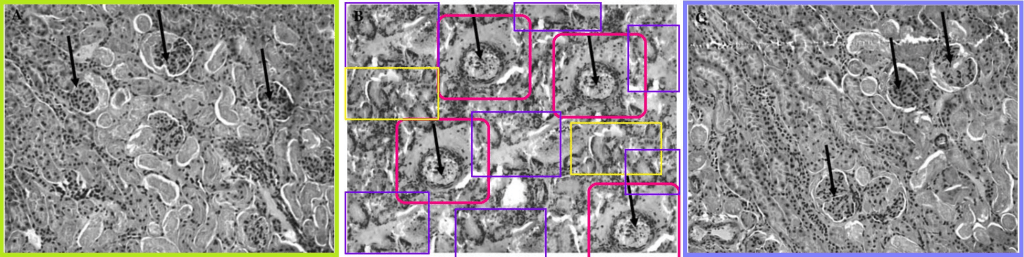
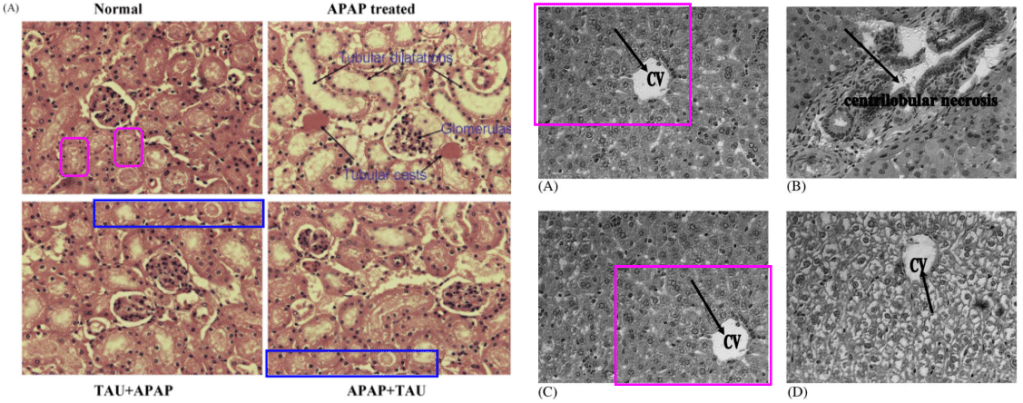
Light green and lavender boxes around the left- and right-hand panels are to call attention for their versatility. Customised with individual manipulations, they appeared in multiple incarnations, illustrating the effect of different toxin / treatment combinations on kidney microstructure. In 2010, for instance, the injuries were caused by Acetaminophen and treated with Arjun-tree squeezings. The colorised versions might even be the originals… no, wait, they’re photoshopped too. Cited 90 times.

In 2009 the injuries were sequelae of Type-1 Diabetes… unless they were caused by old lace arsenic, and treated with taurine. Cited 75 and 29 times.

[right] Fig 3 from “Arjunolic acid: beneficial role in type 1 diabetes and its associated organ pathophysiology” (Manna & Sil 2012).
 As any fule kno, taurine is abundant in human tissues, and synthesised in the liver… though this does not stop people from absorbing vast quantities in energy drinks, as food supplements, and (in the case of Renfield) as taurine-rich spiders, all with no discernable effect. Nor did it stop various numpties from prescribing it to extend the lifespan of mice. Its exact biological role is unclear, which is why Parames Sil and his students decided that it must be an antioxidant, and therefore the ideal treatment for cadmium- or arsenic-poisoning.
As any fule kno, taurine is abundant in human tissues, and synthesised in the liver… though this does not stop people from absorbing vast quantities in energy drinks, as food supplements, and (in the case of Renfield) as taurine-rich spiders, all with no discernable effect. Nor did it stop various numpties from prescribing it to extend the lifespan of mice. Its exact biological role is unclear, which is why Parames Sil and his students decided that it must be an antioxidant, and therefore the ideal treatment for cadmium- or arsenic-poisoning.

Cats lost the pathways for synthesising taurine on account of an all-meat diet that supplies so much. Back in the late 80s, P.D. Pion showed that low-quality taurine-poor cat-kibble was causing heart failure in felines. It follows that feline deficiency might be the cause of dilated cardiomyopathy in taurines though no-one in the subsequent 36 years has conducted any research into this possibility.

Going back to the Arjuna-tree squeezings, Arjunolic acid also proves to be indicated when acetaminophen causes hepatic injury. Paracetamol overdoses are a significant cause of death, but I hope that clinical guidelines aren’t revised to accommodate fraudulent Photoshop festivals like this. 84, then 75 citations.

Reprinted as Fig 5A,B of “Arjunolic acid: A new multifunctional therapeutic promise of alternative medicine” (Ghosh & Sil 2013).
Manna and Sil collated some earlier fake results in a 2012 Review, “Arjunolic acid: beneficial role in type 1 diabetes and its associated organ pathophysiology” (cited 29 times). The result was a target-rich environment. As Fig 2, they reprinted these exuberantly-enhanced images of spleen tissue from 2010 (originally 59 citations).

Figures 4 and 5 – ostensibly slices of heart muscle and liver tissue – were reprinted from 2012 and 2010 respectively (cited 36 and 159 times). And who can begrudge the authors the reuse of images in which they had invested so much effort?
[right] Fig 11A of “Contribution of type 1 diabetes to rat liver dysfunction and cellular damage via activation of NOS, PARP, IκBα/NF-κB, MAPKs, and mitochondria-dependent pathways: Prophylactic role of arjunolic acid” (Manna et al 2010).
Those studies used streptozotocin to destroy pancreas β-cells and induce diabetes in the lab animals. Streptozotocin blurs in my mind with the dromozoan symbiotes from “A Planet called Shayol“, but that’s just me. Evidently if you use alloxan for diabetic induction then taurine is the more suitable treatment – unless it’s Kombucha. Here are some more versatile microphotographs, not of dromozoa.

[right] Fig 4 from “Taurine exerts hypoglycemic effect in alloxan-induced diabetic rats, improves insulin-mediated glucose transport signaling pathway in heart and ameliorates cardiac oxidative stress and apoptosis” (Das et al 2012).
The authors were so proud of this copy-pasta gallimaufrey that they reprinted it in “Mechanism of the protective action of taurine in toxin and drug induced organ pathophysiology and diabetic complications: a review” (Das et al 2012). But its transformations did not end there. WARNING: more Dromozoa!



It’s time to explain. Anti-oxidants are a perennial topic of research in the diet-supplement / wallet-decrement and life-extension industries, for they mop up the Antifa agitators free radicals created by environmental toxins and by normally-functioning midichlorians and mitochondria. In practice they make no difference, but that’s only because the experiments failed; antioxidation itself cannot be failed.
 Naturally this inspired a race to prove that the ingredients of Traditional Herbal Remedies have antioxidising, marketable properties. This is most apparent among Indian scientists (though not limited to them), pandering to the ongoing revival of medieval magic in support of national / cultural pride.
Naturally this inspired a race to prove that the ingredients of Traditional Herbal Remedies have antioxidising, marketable properties. This is most apparent among Indian scientists (though not limited to them), pandering to the ongoing revival of medieval magic in support of national / cultural pride.
So (for instance) poisoning with arsenic, lead or cadmium all turn out to be manifestations of free radicals and Reactive Oxygen Species (ROS); therefore they’re easily controlled, if not with Arjunolic acid then with mango squeezings, or jute leaves (Corchorus olitorius), or Water Spinach (Ipomoea aquatica), or Abroma augusta.*

[right] Fig 7 from “The effects of two common edible herbs, Ipomoea aquatica and Enhydra fluctuans, on cadmium-induced pathophysiology: a focus on oxidative defence and anti-apoptotic mechanism” (Dua et al 2015).
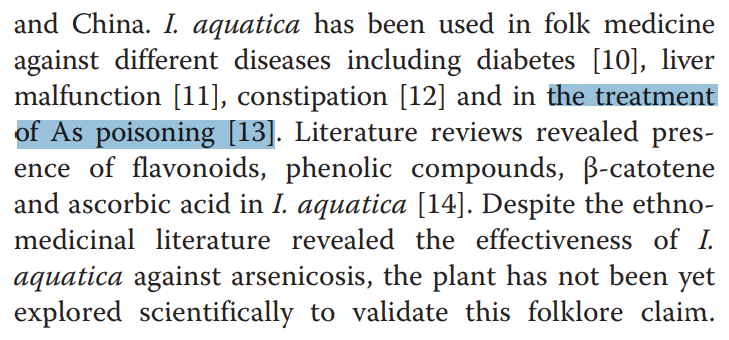
The alternative is to ensure that people have access to arsenic-free drinking water. That would cost money and would not enrich the herbalism industry. It is one of life’s little arsenies ironies that Indian herbal remedies, though widely pimped out as “heavy metal detox”, are more likely to cause arsenicosis and lead / mercury poisoning.
 All this brings me back to Parames C. Sil – doyen of antioxidation, scourge of the ROS, and Senior Professor at the Division of Molecular Medicine of the Bose Institute, which is where the Bosone Layer was discovered. Earlier in his career, Dr Sil studied under one of the high-profile research brigands that US academia spawns with such profligacy, in the Lerner Research Institute in Cleveland. This earned him a minor author slot on a retracted 2004 paper-shaped dumpster from the lab of Subha Sen, who left Cleveland Clinic in 2021 amid fraud findings and retractions.
All this brings me back to Parames C. Sil – doyen of antioxidation, scourge of the ROS, and Senior Professor at the Division of Molecular Medicine of the Bose Institute, which is where the Bosone Layer was discovered. Earlier in his career, Dr Sil studied under one of the high-profile research brigands that US academia spawns with such profligacy, in the Lerner Research Institute in Cleveland. This earned him a minor author slot on a retracted 2004 paper-shaped dumpster from the lab of Subha Sen, who left Cleveland Clinic in 2021 amid fraud findings and retractions.
Sagartirtha Sarkar , Douglas W. Leaman , Sudhiranjan Gupta , Parames Sil , David Young , Annitta Morehead , Debabrata Mukherjee , Norman Ratliff , Yaping Sun , Mary Rayborn , Joe Hollyfield , Subha Sen Cardiac overexpression of myotrophin triggers myocardial hypertrophy and heart failure in transgenic mice Journal of Biological Chemistry (2004) doi: 10.1074/jbc.m308488200
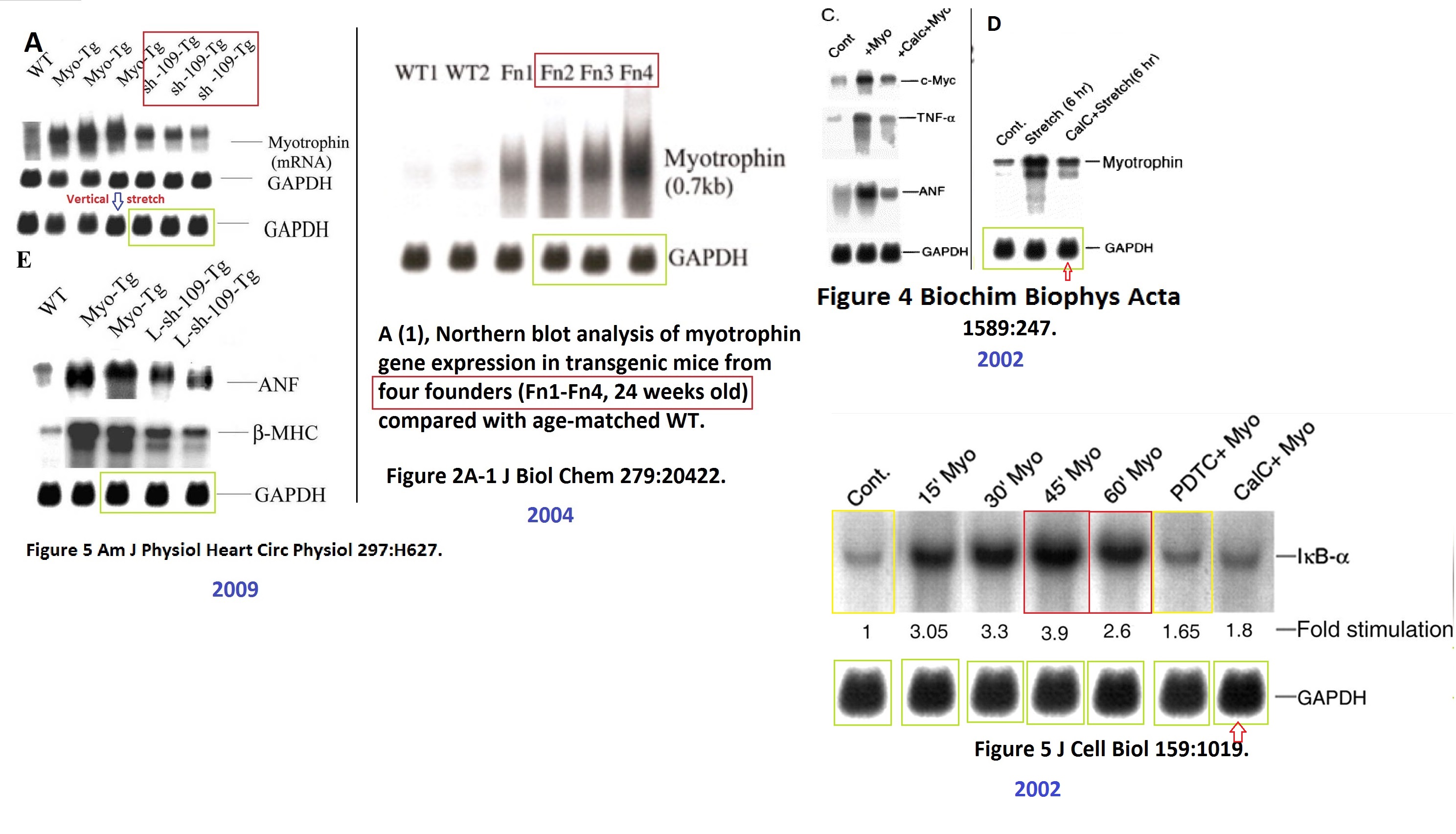
 That was a first step on Sil’s pathway to becoming highly ranked and highly cited within the Indian professoriat establishment,** mentoring a new generation of students, esteemed and sought-after to write well-cited review articles and book chapters in which he cites his own faked papers. His contribution to the sum of human knowledge is substantial but negative.
That was a first step on Sil’s pathway to becoming highly ranked and highly cited within the Indian professoriat establishment,** mentoring a new generation of students, esteemed and sought-after to write well-cited review articles and book chapters in which he cites his own faked papers. His contribution to the sum of human knowledge is substantial but negative.
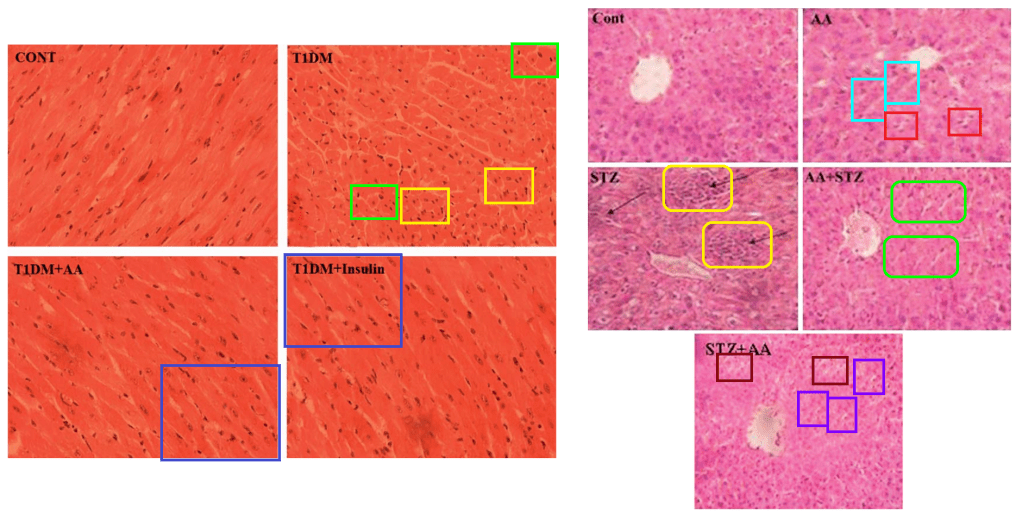
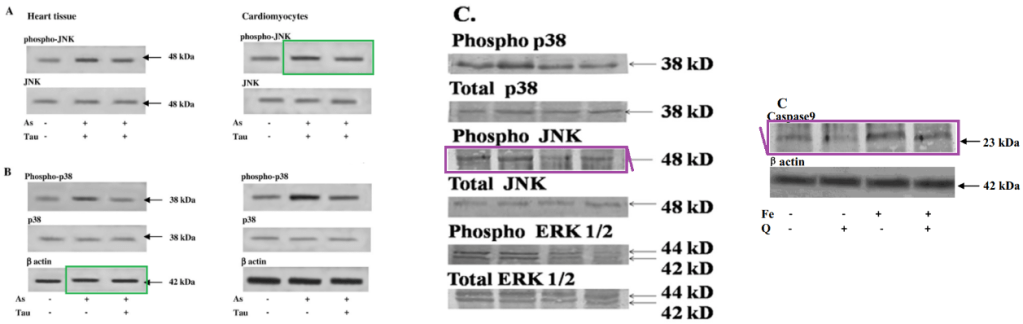
We learn that Dr Sil’s digital wizardry extends to heart muscles, which are among the victims of alloxan-induced diabetes (and of CCl4), although the damage is assuaged by taurine again (and by arjunolic acid).

[right] Fig 5 from “Taurine exerts hypoglycemic effect in alloxan-induced diabetic rats, improves insulin-mediated glucose transport signaling pathway in heart and ameliorates cardiac oxidative stress and apoptosis” (Das et al 2012).
I grudgingly concede that if taurine deficiency causes cardiomyopathy in cats, then it makes perfect sense that doxorubicin-induced cardiomyopathy (in rats) is also a kind of taurine deficiency, easily treated with a combination of dietary supplements and Photoshop.
All that is archival material, though. How about some more recent evidence that Dr Sil’s current students put all their images into a lucky-dip bag and pull things out at random when they make up a story and need illustrations?

[right] Fig 6B from “Tumor targeted delivery of umbelliferone via a smart mesoporous silica nanoparticles controlled-release drug delivery system for increased anticancer efficiency” (Kundu et al 2020).


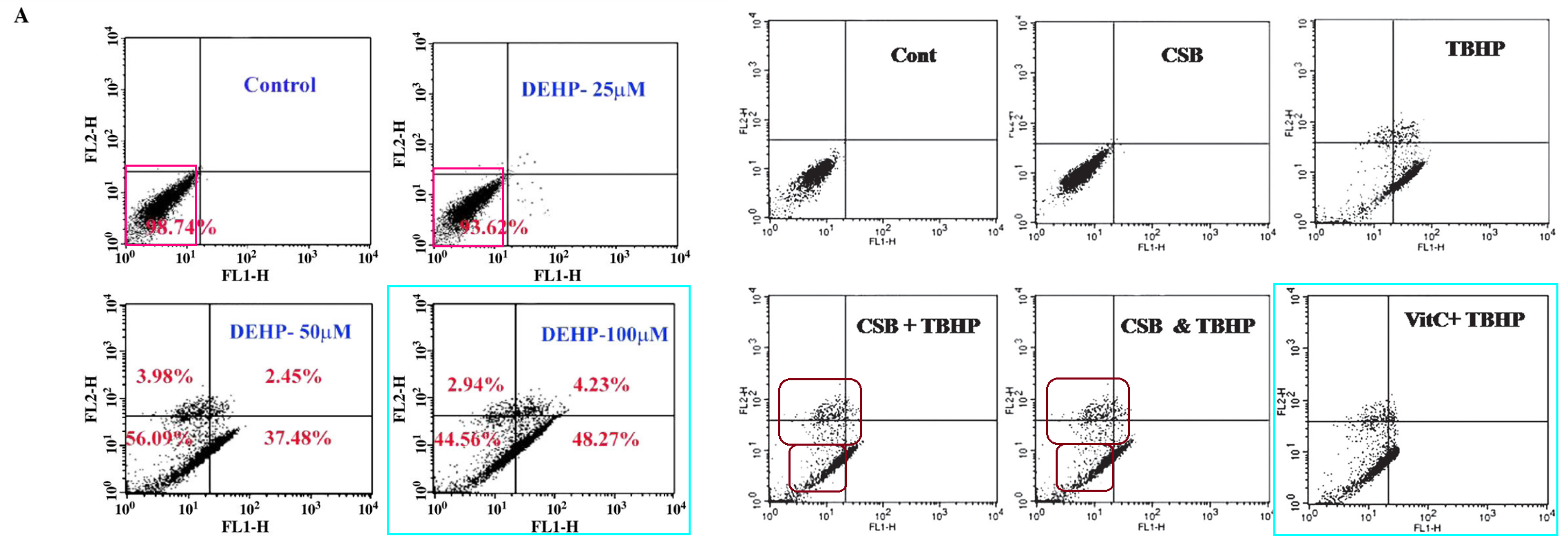
But I would hate for readers to think that the Sil School of Photoshop is limited to microtomed slices of heart or liver or kidneys. If you are like me, you are bored now, and wondering whether Sil’s mad skills with the clone-stamp tool extend to manipulating flow-cytometry apoptosis scatterplots to support a Just-So fable about the Mango-Squeezings and the Toxicity of Lead. HA HA this uncertainty is merely rhetorical; of course they do!
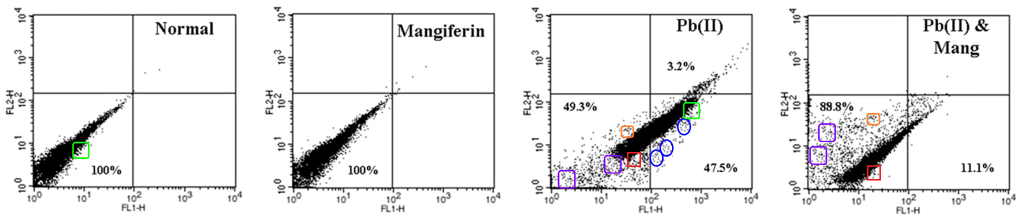
[right] Fig 8 from “Protective role of a coumarin-derived schiff base scaffold against tertiary butyl hydroperoxide (TBHP)-induced oxidative impairment and cell death via MAPKs, NF-κB and mitochondria-dependent pathways” (Ghosh et al 2011)
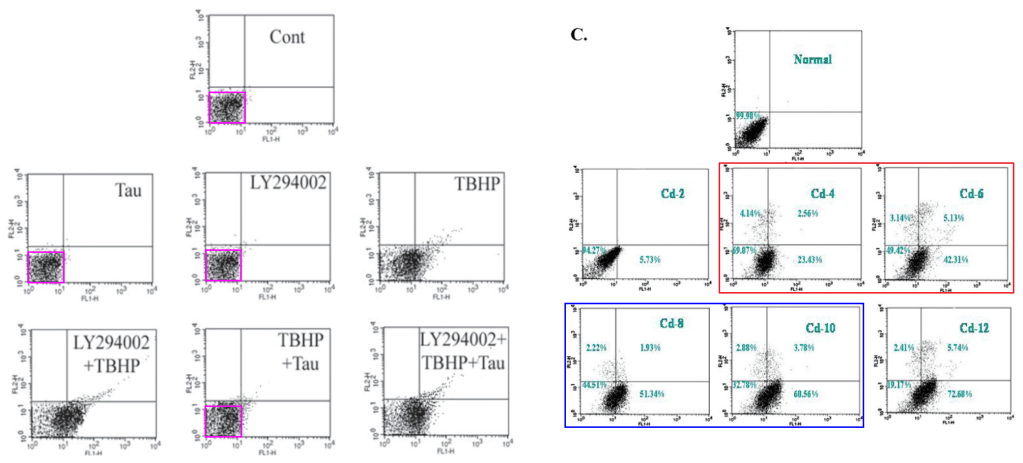
[right] Fig 3 from “Taurine protects murine hepatocytes against oxidative stress-induced apoptosis by tert-butyl hydroperoxide via PI3K/Akt and mitochondrial-dependent pathways” (Roy & Sil 2012).
Here are some cells using immunofluorescence to record their mitochondrial function and survival after different combinations of toxin and treatment. Arjunolic acid comes into the picture, but also glycine.
[right] Fig 2A from “Involvement of both intrinsic and extrinsic pathways in hepatoprotection of arjunolic acid against cadmium induced acute damage in vitro” (Pal et al 2011).
The rectangle of images outlined with bright pink also appears in Fig 2C of “Protective Role of Taurine against Arsenic-Induced Mitochondria-Dependent Hepatic Apoptosis via the Inhibition of PKCδ-JNK Pathway” (Das et al 2010).
The shared rectangle outlined with orange makes multiple cameo appearances.
[right] Fig 4a from “Phytomedicinal Role of Pithecellobium dulce against CCl4-mediated Hepatic Oxidative Impairments and Necrotic Cell Death” (Manna et al 2011).
[left] Fig 2A from “Acetaminophen induced acute liver failure via oxidative stress and JNK activation: Protective role of taurine by the suppression of cytochrome P450 2E1” (Das et al 2010).
[right] Fig 7B from “Taurine protects acetaminophen-induced oxidative damage in mice kidney through APAP urinary excretion and CYP2E1 inactivation” (Das et al 2010).
 To be scrupulously fair, other products of Sil’s laboratory have not been flagged at PubPeer so are not in the inevitable spreadsheet. They may well be unembellished reports of authentic experiments; readers must decide how much trust they deserve. To lean over so far backwards in the pursuit of fairness as to resemble a Limbo dancer, not all of the entries in the spreadsheet involve such egregious creativity. Some concerns are spliced Southern blots, microphotographs with overlapping fields, or duplicated Western Blot bands, which could be honest error.
To be scrupulously fair, other products of Sil’s laboratory have not been flagged at PubPeer so are not in the inevitable spreadsheet. They may well be unembellished reports of authentic experiments; readers must decide how much trust they deserve. To lean over so far backwards in the pursuit of fairness as to resemble a Limbo dancer, not all of the entries in the spreadsheet involve such egregious creativity. Some concerns are spliced Southern blots, microphotographs with overlapping fields, or duplicated Western Blot bands, which could be honest error.
[right] Fig 4 from “Protection of Arsenic-Induced Hepatic Disorder by Arjunolic Acid” (Manna et al 2007)


The duplicated WB bands seem less accidental and are harder to spot when camouflaged by changes in intensity and contrast. Five points to ImageTwin!
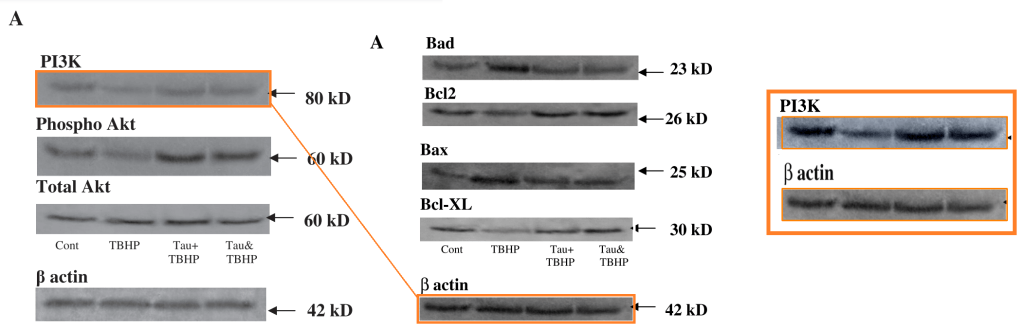
[right] Fig Fig 10C from “Protective effect of arjunolic acid against atorvastatin induced hepatic and renal pathophysiology via MAPK, mitochondria and ER dependent pathways” (Pal et al 2015); and Fig 7C from “Iron oxide nanoparticles mediated cytotoxicity via PI3K/AKT pathway: Role of quercetin” (Sarkar & Sil 2014).
Also less erroneous when they are duplicated between papers, with 180° flips.
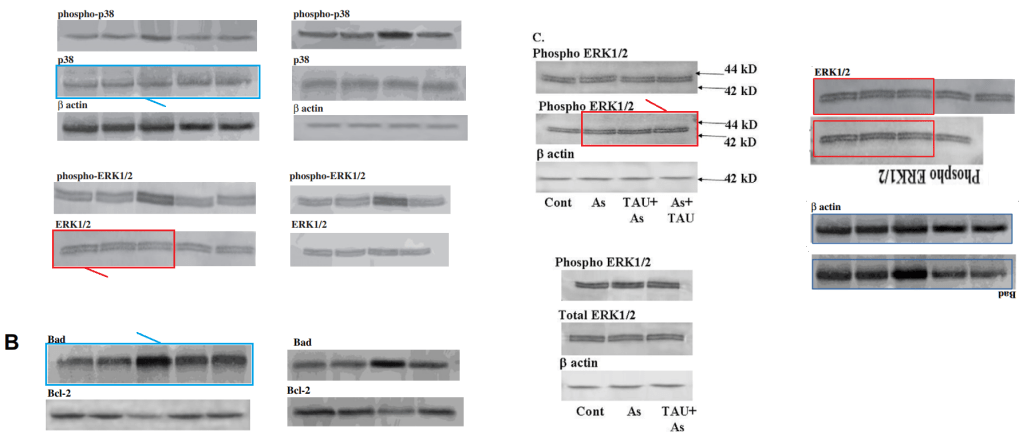
Fig 6C from “Protective Role of Taurine against Arsenic-Induced Mitochondria-Dependent Hepatic Apoptosis via the Inhibition of PKCδ-JNK Pathway” (Das et al 2010).
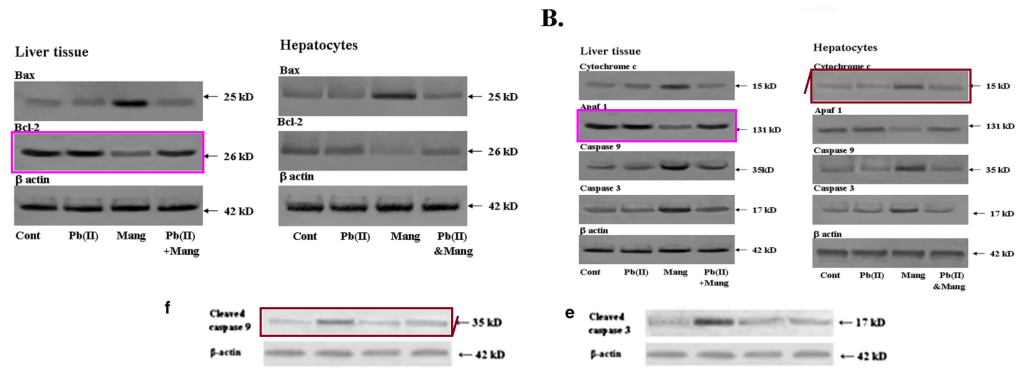
[below] Fig 5f,e from “The effects of two common edible herbs, Ipomoea aquatica and Enhydra fluctuans, on cadmium-induced pathophysiology: a focus on oxidative defence and anti-apoptotic mechanism” (Dua et al 2015).

[right] Fig 4A from “Abroma augusta L. (Malvaceae) leaf extract attenuates diabetes induced nephropathy and cardiomyopathy via inhibition of oxidative stress and inflammatory response” (Khanra et al 2015).
 In the last two cases, the duplications are not only external to papers, but bring in the work of an unrelated group at the Advanced Pharmacognosy Research Laboratory at Jadavpur University (Kolkata) who have their own herbal-antioxidant agenda. The group – Tarun K. Dua and Ritu Khanra inter alia – centres around Saikat Dewanjee. Dewanjee’s oeuvre is smaller but equally fictitious, so it receives a separate sheet in the spreadsheet.
In the last two cases, the duplications are not only external to papers, but bring in the work of an unrelated group at the Advanced Pharmacognosy Research Laboratory at Jadavpur University (Kolkata) who have their own herbal-antioxidant agenda. The group – Tarun K. Dua and Ritu Khanra inter alia – centres around Saikat Dewanjee. Dewanjee’s oeuvre is smaller but equally fictitious, so it receives a separate sheet in the spreadsheet.
How the crossover occurred is anyone’s guess. It is as if visual data want to be free, and images want to migrate from one laboratory to another like asylum-seekers fleeing persecution in their homelands. “The Migration of Symbols” is not just a collection of essays by Rudolf Wittkower!
As with Sil’s group, Dewanjee and his colleagues display a chronic failure to distinguish between arsenic, cadmium and lead. Perhaps heavy-metal poisoning from self-administered ayurvedic remedies has compromised their faculties.

Fig 9 from “Protective effect of Corchorus olitorius leaves against arsenic-induced oxidative stress in rat brain” (Das et al 2010).
Fig 17 from “Toxic effects of lead exposure in Wistar rats: Involvement of oxidative stress and the beneficial role of edible jute (Corchorus olitorius) leaves” (Dewanjee et al 2013).
The converse confusions, between squeezings of different plants, also occur.

[right] Fig 11A,B from “Water Spinach, Ipomoea aquatica (Convolvulaceae), Ameliorates Lead Toxicity by Inhibiting Oxidative Stress and Apoptosis” (Dewanjee et al 2015).
Not so invested in the dark arts of manipulation, Dewanjee et al are content to recycle images. Here is Fig 12 of “Wheat phenolics suppress doxorubicin-induced cardiotoxicity via inhibition of oxidative stress, MAP kinase activation, NF-κB pathway, PI3K/Akt/mTOR impairment, and cardiac apoptosis” (Sahu et al 2019).

The overlaps among panels extend to Fig 6B from “Abroma augusta L. (Malvaceae) leaf extract attenuates diabetes induced nephropathy and cardiomyopathy via inhibition of oxidative stress and inflammatory response” (Khanra et al 2015)…

… and to Fig 10A of “Sansevieria roxburghiana Schult. & Schult. F. (Family: Asparagaceae) Attenuates Type 2 Diabetes and Its Associated Cardiomyopathy” (Bhattacharjee et al 2016).

But wait, there’s more! The management apologises for the garish palette.


[below] Fig 13b from “Myricitrin, a Glycosyloxyflavone in Myrica esculenta Bark Ameliorates Diabetic Nephropathy via Improving Glycemic Status, Reducing Oxidative Stress, and Suppressing Inflammation” (Dua et al 2021}
The group have their own favorite slides of immunofluorescing cells, suitable for any occasion.

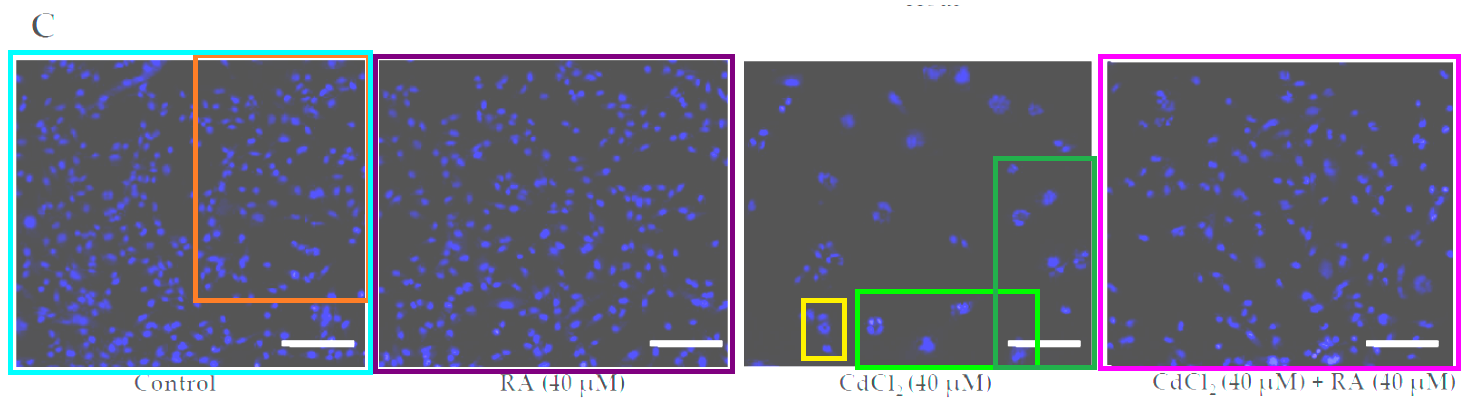


Fig 1C from “Carnosic Acid Attenuates Cadmium Induced Nephrotoxicity by Inhibiting Oxidative Stress, Promoting Nrf2/HO-1 Signalling and Impairing TGF-β1/Smad/Collagen IV Signalling” (Das et al 2019).
Fig 3e from “The effects of two common edible herbs, Ipomoea aquatica and Enhydra fluctuans, on cadmium-induced pathophysiology: a focus on oxidative defence and anti-apoptotic mechanism” (Dua et al 2015).

Their supplies of Western Blot bands are untethered from specific experiments.
Fig 3 from “Protocatechuic Acid, a Phenolic from Sansevieria roxburghiana Leaves, Suppresses Diabetic Cardiomyopathy via Stimulating Glucose Metabolism, Ameliorating Oxidative Stress, and Inhibiting Inflammation” (Bhattacharjee et al 2017).
Fig 5 from “Carnosic Acid, a Natural Diterpene, Attenuates Arsenic-Induced Hepatotoxicity via Reducing Oxidative Stress, MAPK Activation, and Apoptotic Cell Death Pathway” (Das et al 2018).
WB bands submerge like U-boats and resurface elsewhere. Tracing their repeat appearances across papers requires large sprawling files, and ideally would involve cotton threads in multiple colors.
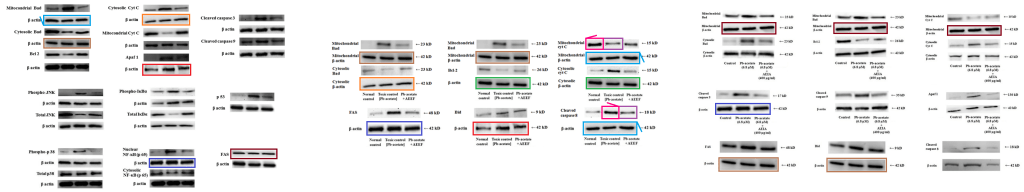
Figs 4 and 5 from “Cytoprotective and Antioxidant Effects of an Edible Herb, Enhydra fluctuans Lour. (Asteraceae), against Experimentally Induced Lead Acetate Intoxication” (Dua et al 2016).
Figs 3 and 4 from “Water Spinach, Ipomoea aquatica (Convolvulaceae), Ameliorates Lead Toxicity by Inhibiting Oxidative Stress and Apoptosis” (Dewanjee et al 2015).
Also there is the problem of running out of colors. So I will stop after these two.
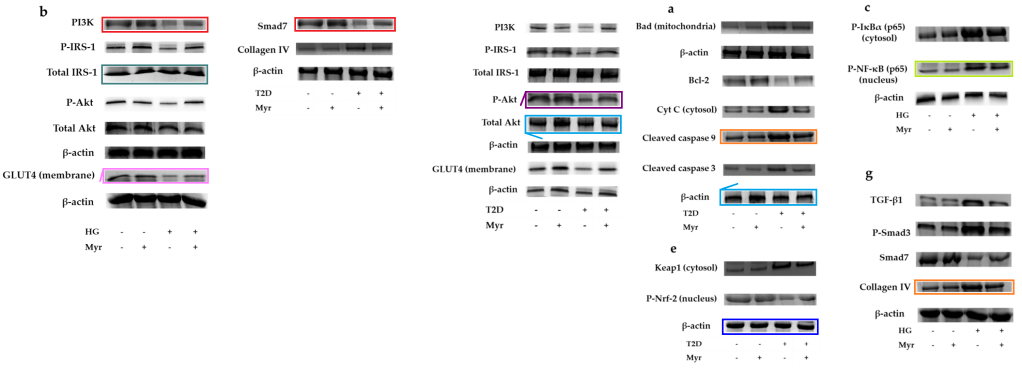
“Carnosic acid attenuates doxorubicin-induced cardiotoxicity” is especially intriguing because Dewanjee is only the second author. In the next example he’s only the penultimate author. In both cases, the main author is a certain Parames C. Sil. Isn’t it satisfying how the scriptwriters have brought the two story-lines together?

One regular co-author in Dewanjee’s fictional oeuvre is Vincenzo De Feo – a Professor in the Pharmacy Faculty of Università degli Studi di Salerno (UNISA). De Feo has 362 publications in his ResearchGate entry so I guess he can’t be too finicky when deciding which to sign. I will leave any further inquiries to Leonid.
[Prof De Feo chose not to reply to emails- LS]
I must credit a series of single-use PubPeer nyms who started this line of inquiry by documenting Sil’s and Dewanjee’s illustrative skills: Bruceia pulverina, Dasytes fusculus, Dissodinium pseudolunula, Drosophila sordidula, Dumasia truncata, Fluminicola scopulinus, Hydroptila banmaekap and others. Also Kaveh Bazargan who wondered [pers. comm.] if more shoes remained un-dropped.
* “In this issue, the World Health Organization (WHO) also recommended the ingestion of β-carotene, ascorbic acid, tocopherol and/or trace elements like Zn and Se to reverse symptoms of arsenicosis [9].” Um, no, what G. Howard wrote two decades ago was a recommendation to WHO.
- Howard G. Arsenic, drinking-water and health risk substitution in arsenic mitigation: a discussion paper. Geneva: World Health Organization; 2003. WHO/SDE/WSH/03.06; http://www.who.int/water_sanitation_health/dwq/wsh0306/en/index.html.
** The Indian academic establishment includes Rashmi Madhuri and Prashant Sharma, and their gurus Bhim Bali Prasad and Avinash C. Pandey. The impostures from the first two were so blagrant (a word I made up just now out of ‘blatant’ and ‘flagrant’) that their students rioted, colleagues wrote a petition, and an Inquiry was announced. That inquiry’s 2018 report was delayed on account of COVID-19 until its postponement became permanent suppression, while Madhuri and Sharma continue their careers with better-disguised forgeries. Sylvain Bernès remarked just the other day about the many Sharma-Madhuri atrocities that remain unretracted and continue to accrue citations.

My expectations of a response from the Bose Institute are not high.

Donate to Smut Clyde!
If you liked Smut Clyde’s work, you can leave here a small tip of 10 NZD (USD 7). Or several of small tips, just increase the amount as you like (2x=NZD 20; 5x=NZD 50). Your donation will go straight to Smut Clyde’s beer fund.
NZ$10.00










It’s a remarkably formulaic “research” process. If it weren’t for all the photoshopped images these sort of papers would be unbearably boring. This is also a favourite structure for Malaysian researchers.
LikeLike
The departments of Pharmacology and Toxicology at universities should be closed / ended all over the world. The percentage of fraud and nonsense and quackery in these fields is unmatchable. Enough is enough.
LikeLike
You are correct about pharmacology. See the case of pharmacology professor Anupam Bishayee who got away with “misconduct”. Dr. Helen Hill wrote a book about it:
“Hidden Data: The Blind Eye of Science”
LikeLiked by 1 person