The medically-trained pharmacologist Domenico Pratico is professor at the Lewis Katz School of Medicine at Temple University in Philadelphia, USA, and a patriotic Italian. His research regularly proves that olive oil is capable of miracle healing of brain diseases, while other plant oils, like canola, are simply poison. Earlier this year, three whistleblowers raised concerns about Pratico’s data with the Office of Research Integrity (ORI), who then forwarded the evidence to Temple University for investigation. Who then diligently wiped their arses with it, while issuing one press release after another about what genius Professor Pratico is, and what amazing cures against Alzheimer’s and other brain diseases he has developed. The funding money flows like olive oil.
Just days ago, Pratico was awarded a governmental grant of $3.8 Million, for clinical research. Which means: Pratico’s questionable preclinical work, which Temple University refused to investigate, will now translate into human experimenting. Porca miseria, eh?
On the occasion of the $3.8 million cash shower, Pratico was quoted in the Temple announcement:
“We are very excited to have the opportunity, through funding from the Pennsylvania Department of Health, to investigate the effects of cardiovascular risk factors on the development of Alzheimer’s pathology […] “By bringing basic research from preclinical studies together with clinical investigation, we can gain significant insight into how neurovascular dysfunction correlates with cognitive outcome in Alzheimer’s disease“.
For the Temple University, human patients are apparently just as a replaceable commodity as Pratico’s olive-oil fed mice, important only is that they generate money. Here are the whistleblower reports to ORI, which I obtained from an inside source:

Amazing water maze
The analysis of Pratico’s work was done by 3 scientists, but Temple University did not take their concerns seriously. There was also a “Signed statement echoing similar concerns from Dr. Richard Morris, who invented the Morris water maze in 1980s“, which is genuine but I however cannot share it at this stage.
Among other things, the Morris water maze tests were criticised for
- “Unrealistically small error bars”,
- incompatibility of interpreted results with published data,
- fudged statistics,
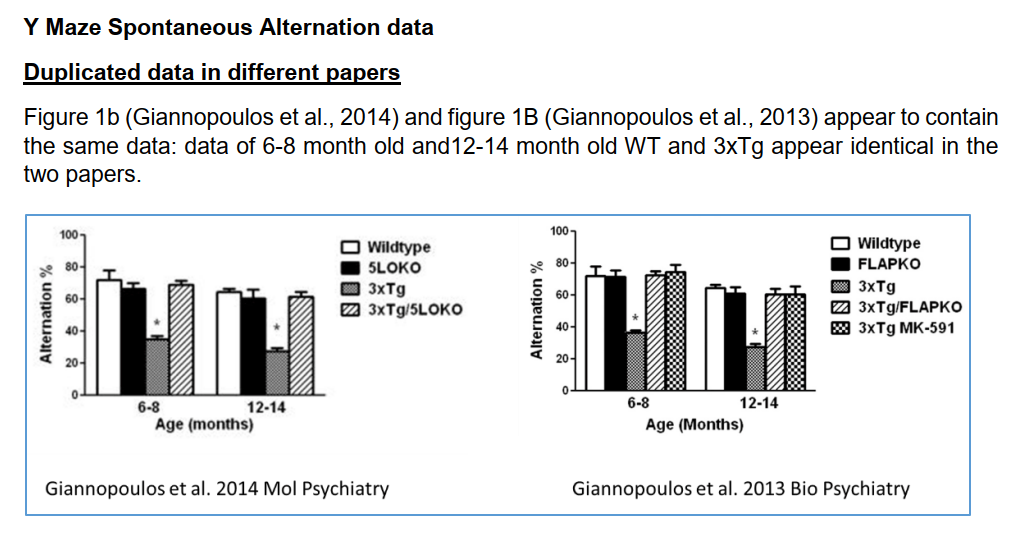
- identical day 4 training results between different mice in 4 different papers,
- almost identical wildtype data between differently aged mice in 3 different papers,
- “Mathematically unsound data“, where half of a 20-mice strong group “would have zero alternation“,
- the very real possibility that the analysed transgenic mice were literally blind (due to a genetic defect), which most certainly would negatively affect their Morris water maze performance,
- “Questionable probe trial latency data“, where Pratico’s mice proved to be cognitively very uniform and otherwise peculiar, with near-identical and surprisingly straight learning curves. Basically, either the mice used to serve in Italian military and marched to Pratico’s commands, or something else was going on.
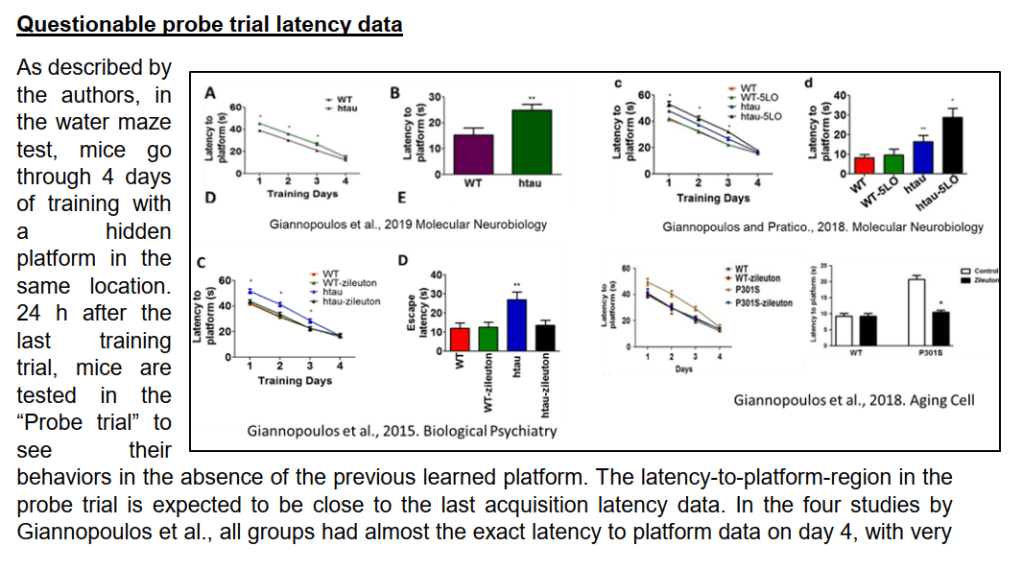
acquisition data (line graphs), the probe trial latency data are highly unexpected.”
All that kind of reminds me of another Italian neuroscientist with leaky water maze data, Elisabeta Ciani of University of Bologna. I think she and Pratico must meet and exchange notes or whatever, although they probably already did. I also fear that Temple University will do what University of Bologna did and blame the poor mice alone for everything.
The water maze data was from the whistleblower report from February 2020. It was followed by another report from June 2020, where actual image data recycling across several papers was flagged. Still, nobody was interested. Meanwhile, screenshots from that report have been now posted on PubPeer, to accompany further evidence of data fudging.

Giannopoulos et al
The papers flagged in the second whistleblower report are mostly about the Leukotriene/5-lypoxigenase targeting therapy of brain diseases, something Pratico published lots about. The mice were treated with the asthma drug Zileuton, which inhibits the 5-lipoxigenase enzyme and thus leukotriene synthesis, and lo and behold, Alzheimer’s disease was cured (IN MICE). Will it translate to humans, or are mice just too different from us biologically, as scientists keep preaching? But what if I told you that it is not the mice who are to blame, but dishonest mouse researchers armed with Photoshop and ruthless determination?
The following papers all have the same common first author, another Mediterranean gentleman of science. The first paper by Giannopoulos et al we shall discuss, is brand new.
Phillip F. Giannopoulos, Jian Chiu, Domenico Praticò Learning Impairments, Memory Deficits, and Neuropathology in Aged Tau Transgenic Mice Are Dependent on Leukotrienes Biosynthesis: Role of the cdk5 Kinase Pathway Molecular Neurobiology (2019) doi: 10.1007/s12035-018-1124-7


Pratico announced human clinical trials: “The research could soon be translated to the clinic, to human patients with Alzheimer’s disease.“
When that paper was published, Pratico was also quoted in the local Philly news:
“It’s very, very exciting,” Praticò said of the study results. “This could be the first time that we could do a treatment.”
The mouse healer can’t wait to start experimenting on real patients, and now he has a $3.8 million grant to do just that. But now, to the next act of this Greek-Italian tragedy. Data from that same groundbreaking Mol Neurobiol 2019 paper was previously published in:
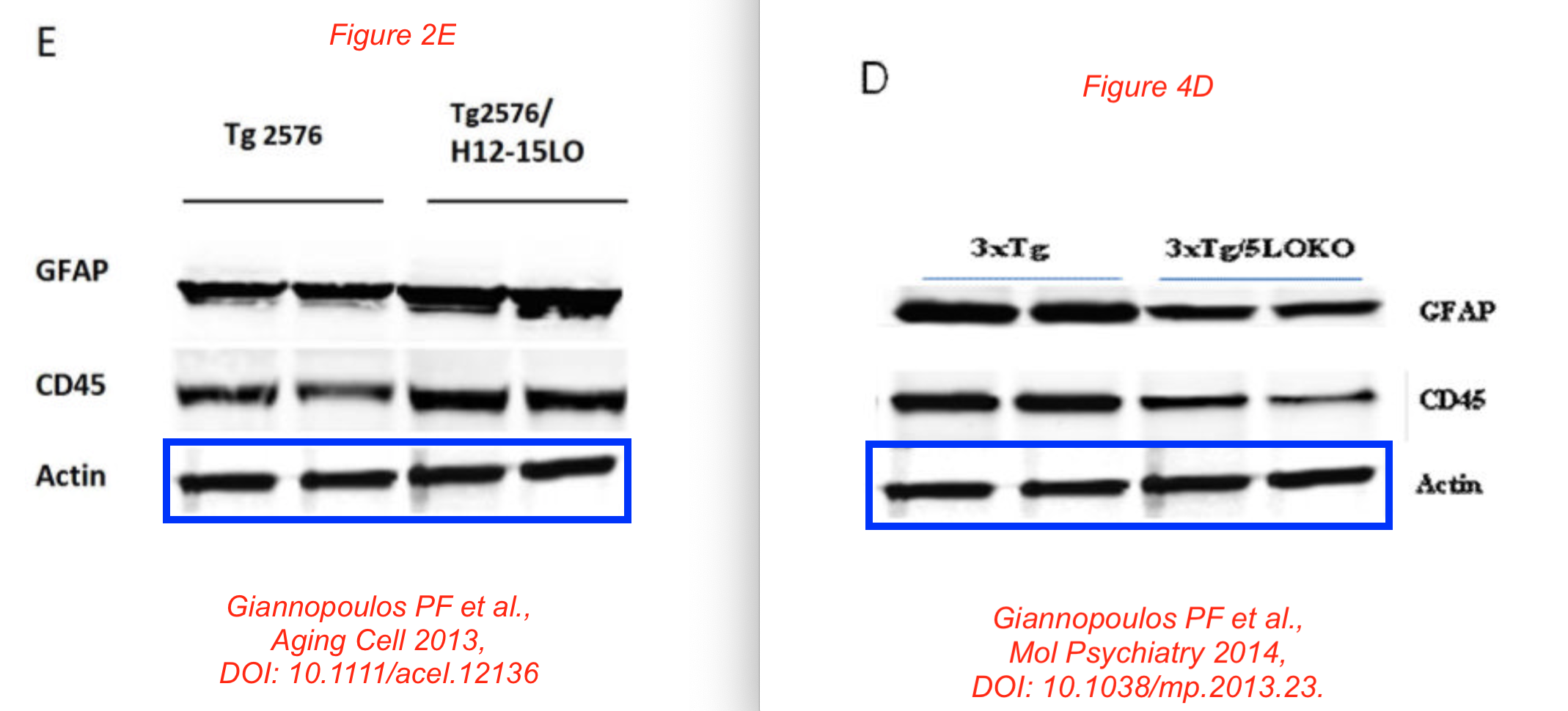
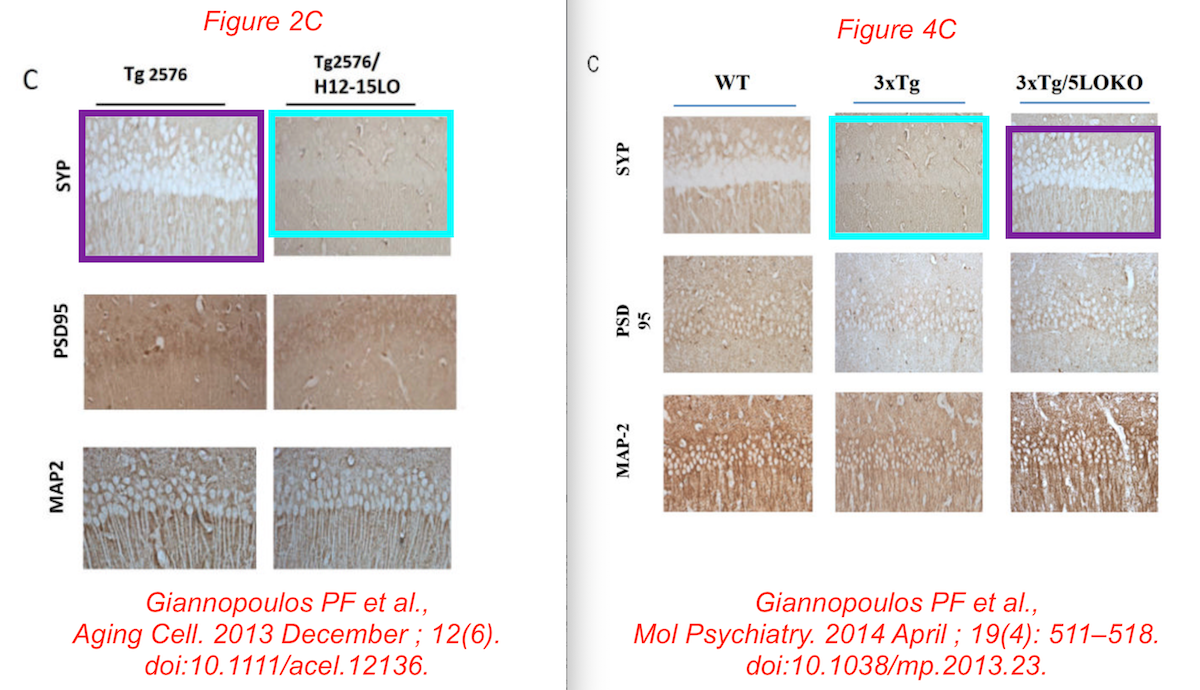
Phillip F. Giannopoulos, Jin Chu, Margaret Sperow, Jian-Guo Li, W. Haung Yu, Lynn G. Kirby, Mary Abood, Domenico Praticò Pharmacologic inhibition of 5-lipoxygenase improves memory, rescues synaptic dysfunction, and ameliorates tau pathology in a transgenic model of tauopathy Biological Psychiatry (2015) doi: 10.1016/j.biopsych.2015.01.015
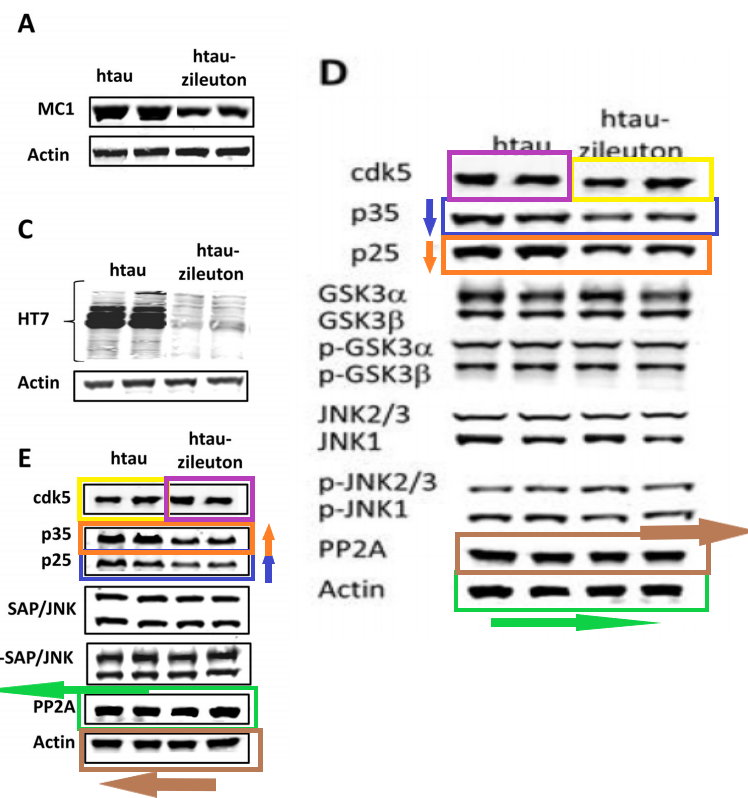
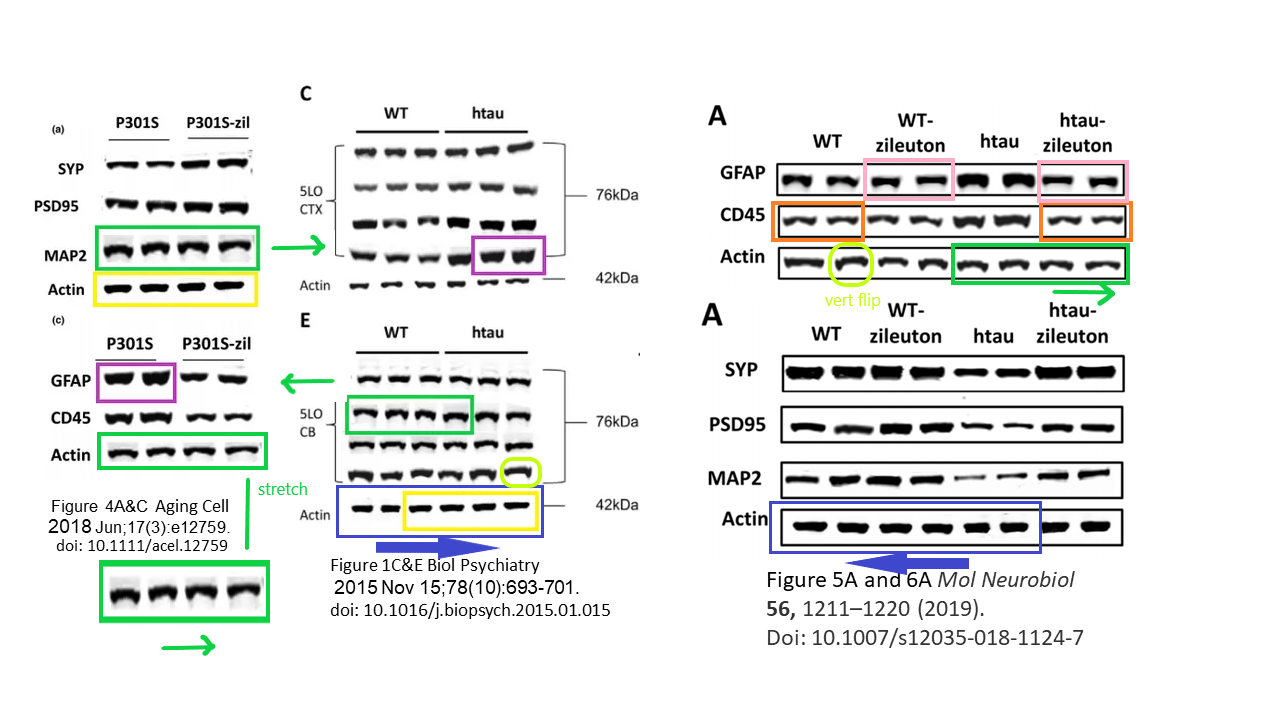
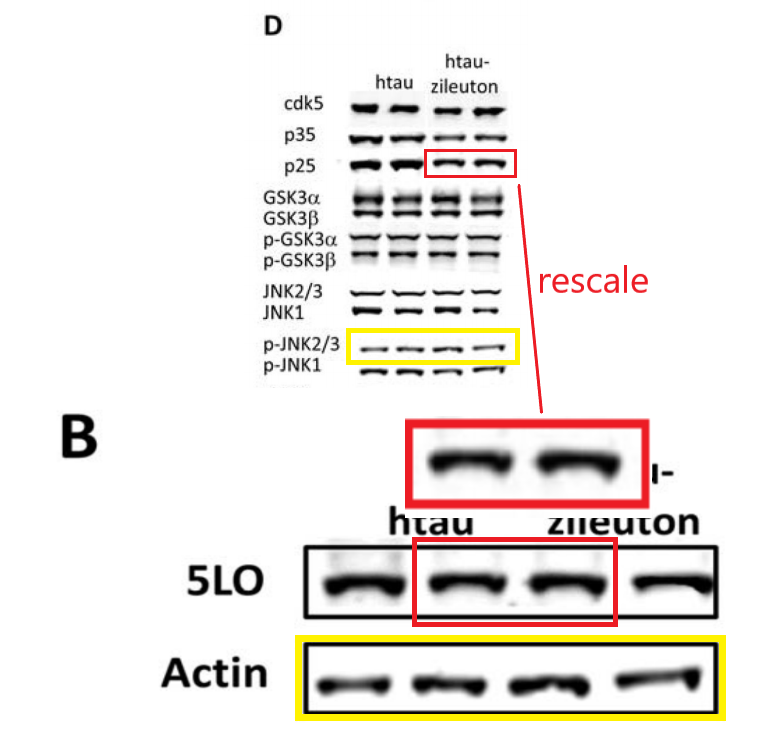
Figure 3B from Mol Neurobiol 2019 vs Figure 4D from Biol Psychiatry 2015
These colourised western blots are really cheerful. In particular, because Temple University proudly announced in a press release that they have a cure for Alzheimer’s with that Mol Neurobiol 2019 paper:
“Inflammation was completely gone from tau mice treated with the drug,” Dr. Praticò said. “The therapy shut down inflammatory processes in the brain, allowing the tau damage to be reversed.”
The study is especially exciting because zileuton is already approved by the Food and Drug Administration for the treatment of asthma. “Leukotrienes are in the lungs and the brain, but we now know that in addition to their functional role in asthma, they also have a functional role in dementia,” Dr. Praticò explained.”
Next time you wonder why mouse research does not translate to humans, don’t blame mice or evolution or a God of your choice, don’t call for mass-scale primate research, but consider starting with scientists like Pratico.
What if I told you the blots from Biol Psychiatry 2015 resurfaced again in a different Giannopoulos paper, again for utterly different experiments with genetically different mice?
Phillip F. Giannopoulos , Jian Chiu , Domenico Praticò Antileukotriene therapy by reducing tau phosphorylation improves synaptic integrity and cognition of P301S transgenic mice Aging Cell (2018) doi: 10.1111/acel.12759
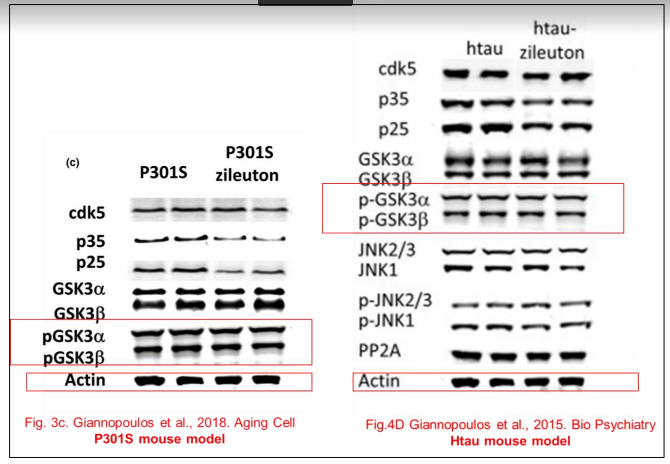
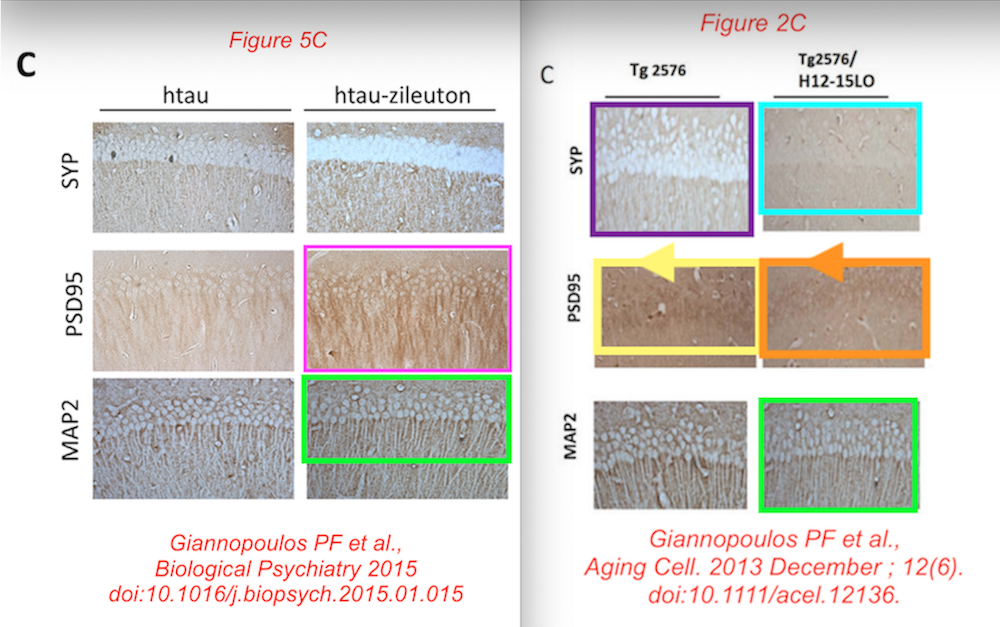
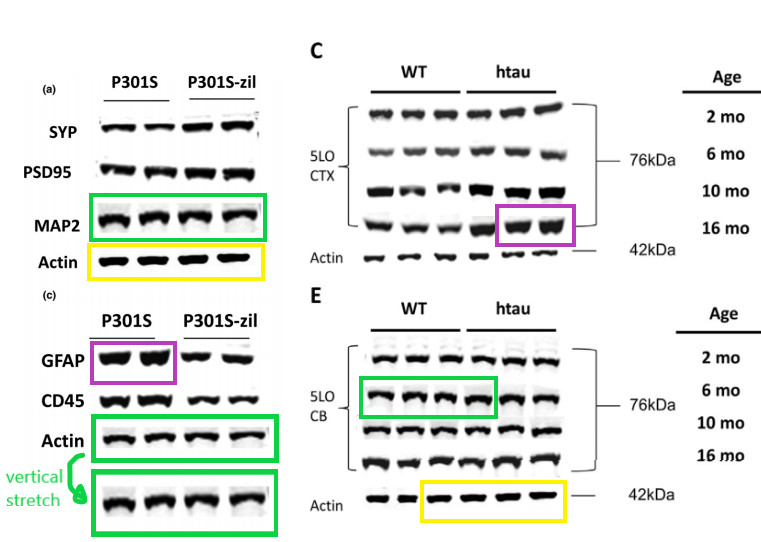

All this, according to Temple University, does not affect the conclusions that they just made almost $4 million dollars with this kind of research. Moving on: the data from that Biol Psychiatry 2015 paper was of course used before, in this publication:
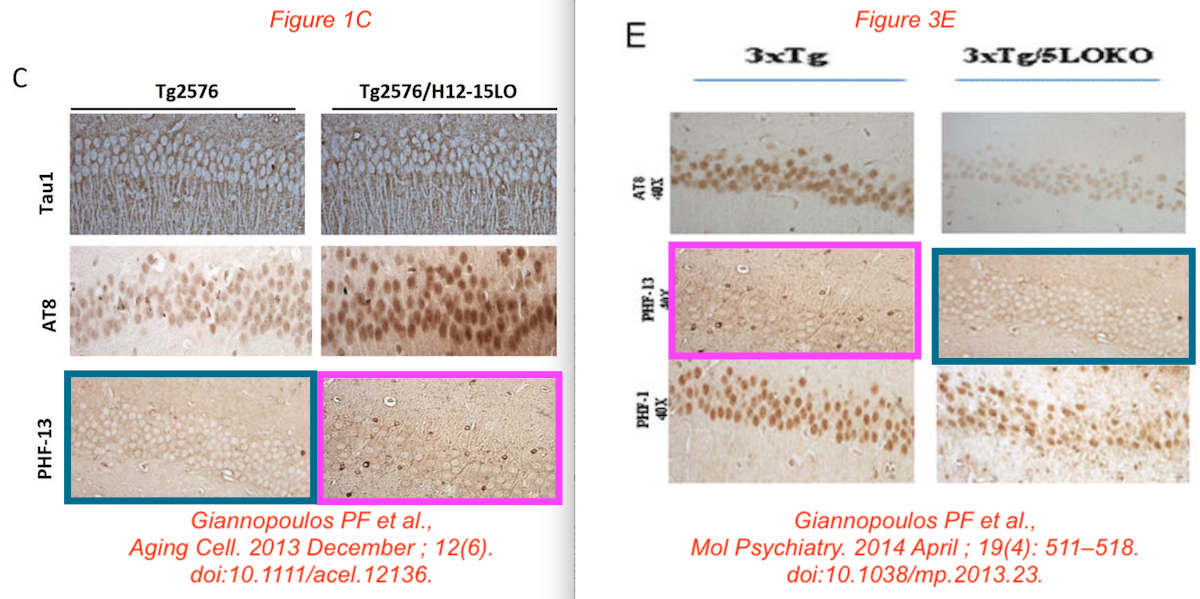
Phillip F. Giannopoulos , Yash B. Joshi , Jin Chu , Domenico Praticò The 12-15-lipoxygenase is a modulator of Alzheimer’s-related tau pathology in vivo Aging Cell (2013) doi: 10.1111/acel.12136


Another paper, featuring same names, claimed to fix Alzheimer’s via leukotriene pathway. It used those same transgenic mice which behaved so uniformly weird in water maze tests.
Phillip F. Giannopoulos, Jin Chu, Yash B. Joshi, Margaret Sperow, Jin-Guo Li, Lynn G. Kirby, Domenico Praticò 5-Lipoxygenase Activating Protein Reduction Ameliorates Cognitive Deficit, Synaptic Dysfunction, and Neuropathology in a Mouse Model of Alzheimer’s Disease Biological Psychiatry (2013) doi: 10.1016/j.biopsych.2013.04.009



And then Elisabeth Bik extended the findings into an area where the Pratico lab starts to look like a clown circus show, with pratfalls, butt-kicks and cake-in-face throwing. Meanwhile, Pratico and his colleagues are about to progress with their hilarious pranks from mice to patients. Hahahahahaha.
Here an example of Pratico’s Alzheimer’s research flagged by Bik:
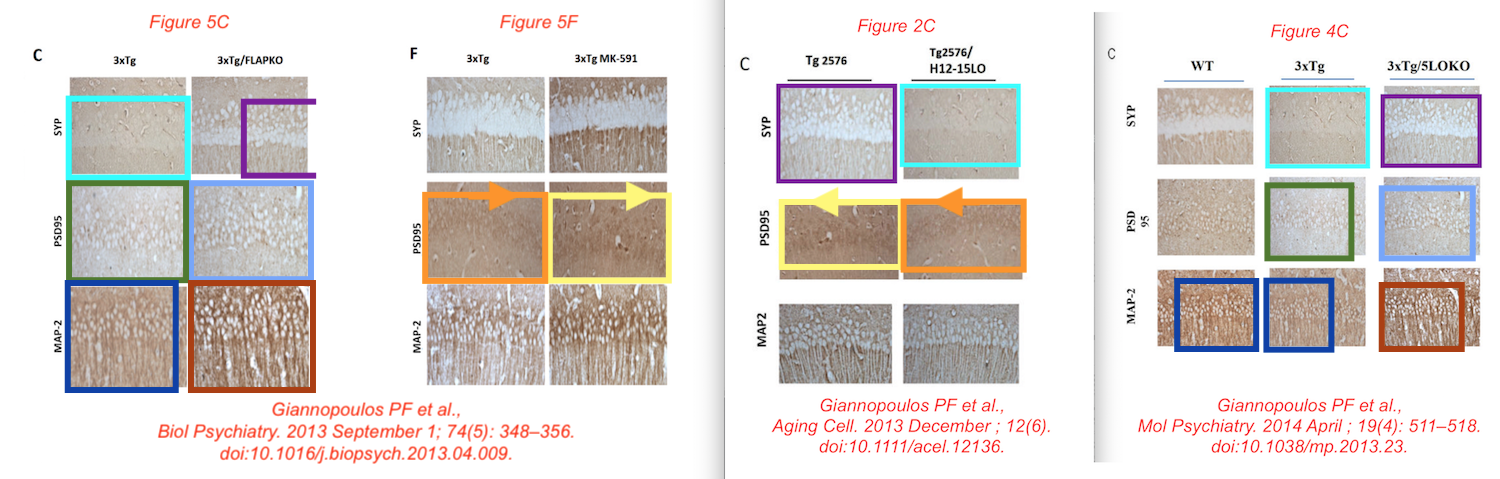
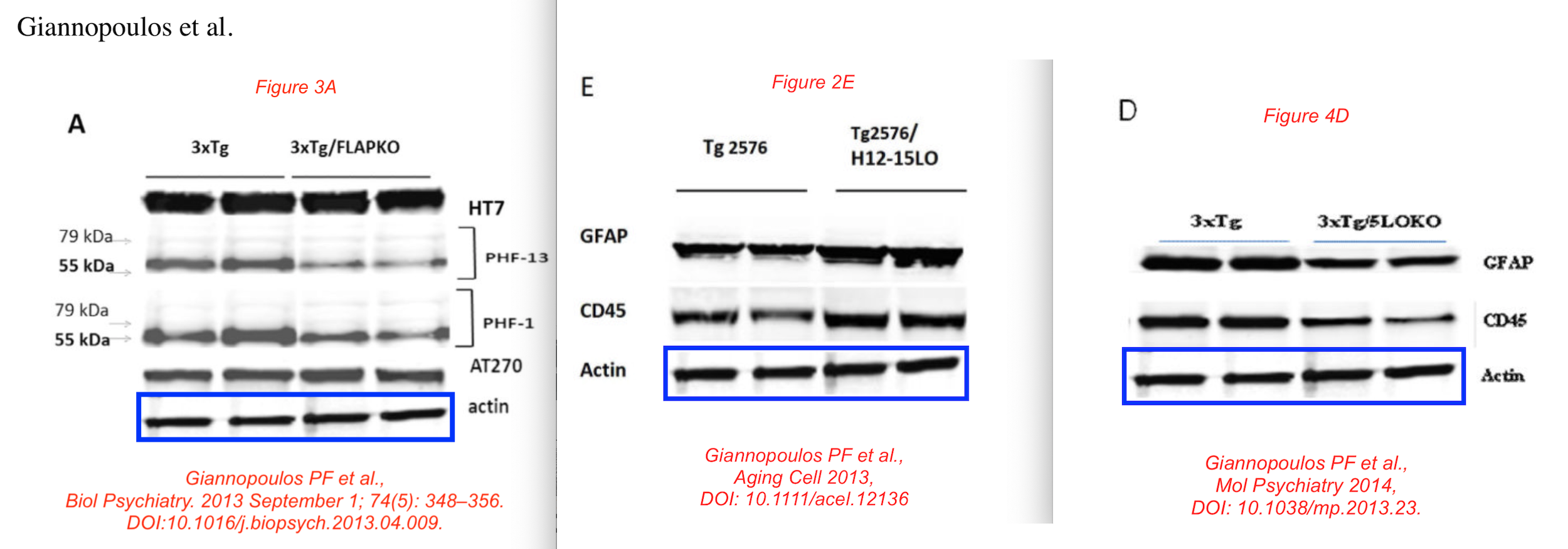
Data reused across thee different papers, in different context, for genetically different mice. You recognise the Giannopoulos Aging Cell 2013 paper, but there are the other two:
P F Giannopoulos, J Chu, Y B Joshi, M Sperow, J-G Li, L G Kirby, D Praticò Gene knockout of 5-lipoxygenase rescues synaptic dysfunction and improves memory in the triple-transgenic model of Alzheimer’s disease Molecular Psychiatry (2014) doi: 10.1038/mp.2013.23
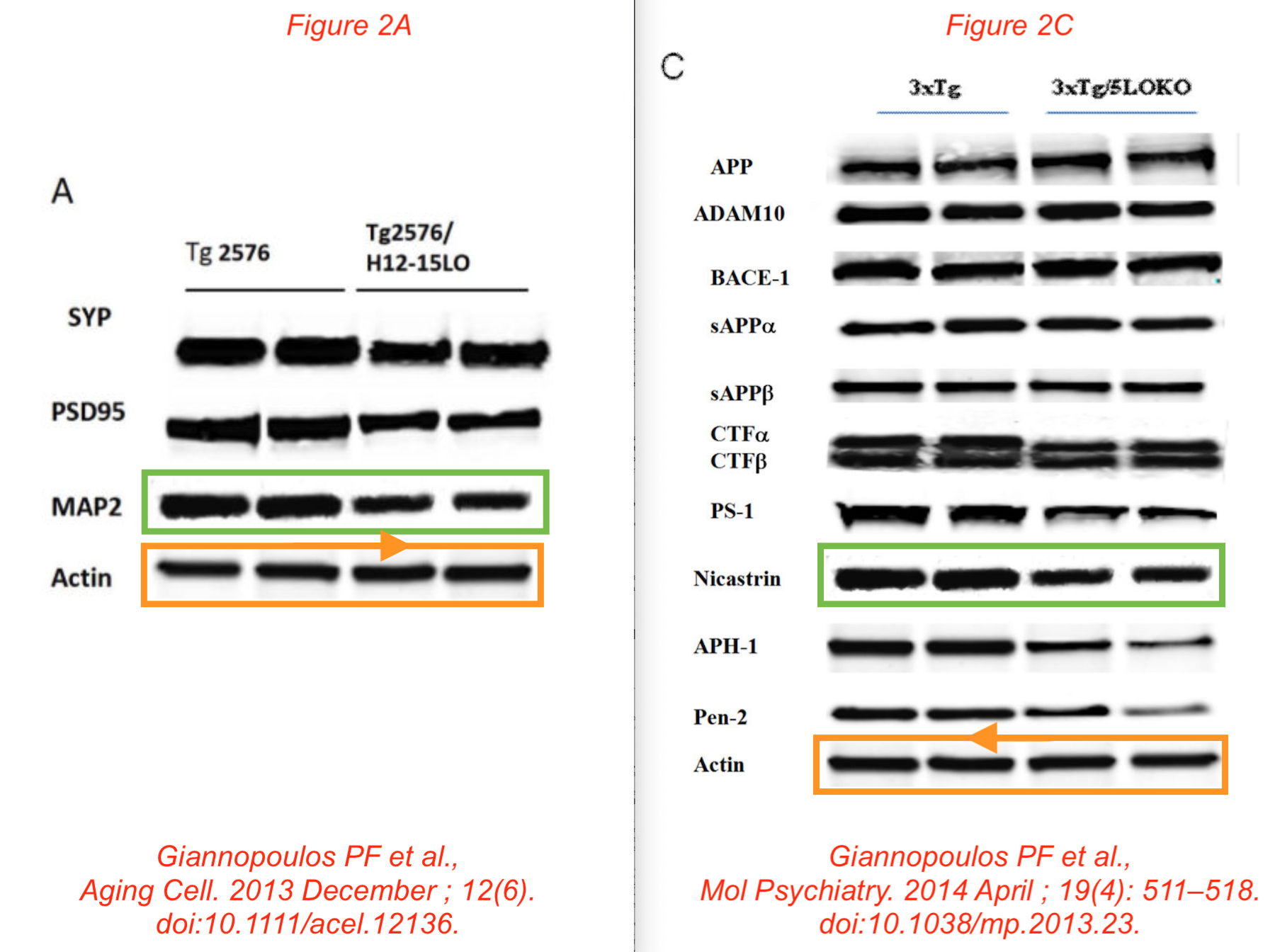
Phillip Giannopoulos is presently Medical Director at a marketing agency. The man sure knows how to unscrupulously market stuff. Before we move to olive oil, have some more of Giannopolos and Pratico:
Phillip F. Giannopoulos, Domenico Praticò Overexpression of 5-Lipoxygenase Worsens the Phenotype of a Mouse Model of Tauopathy Molecular Neurobiology (2018) doi: 10.1007/s12035-017-0817-7






You might say, so what, Mr Giannopoulos might have ACCIDENTALLY used his old research results as representative stand-ins for his predicted new results. But some of the data from that Mol Neurobiol 2018 paper was first published in two other papers from Pratico lab, where Giannapoulos is not a coauthor.
Jian-Guo Li , Carlos Barrero , Salim Merali, Domenico Praticò Five lipoxygenase hypomethylation mediates the homocysteine effect on Alzheimer’s phenotype Scientific reports (2017) doi: 10.1038/srep46002
Jian-Guo Li, Jin Chu, Carlos Barrero, Salim Merali, Domenico Praticò Homocysteine exacerbates β-amyloid pathology, tau pathology, and cognitive deficit in a mouse model of Alzheimer disease with plaques and tangles Annals of Neurology (2014) doi: 10.1002/ana.24145




Olive Oil Healing
Of course someone creative like Pratico will not be stuck with peddling some boring old asthma drug. Media and food industry loves health news about superfoods, and the Italian doctor did not disappoint. In summer 2017, the AAAS science news website EurekaAlert! titled:
“Temple study: Extra-virgin olive oil preserves memory & protects brain against Alzheimer’s”
Pratico explained: “We found that olive oil reduces brain inflammation but most importantly activates a process known as autophagy“, and added: “compared to mice on a regular diet, brain cells from animals in the olive oil group showed a dramatic increase in nerve cell autophagy activation, which was ultimately responsible for the reduction in levels of amyloid plaques and phosphorylated tau.”
The paper was this one:
Elisabetta Lauretti, Luigi Iuliano, Domenico Praticò Extra-virgin olive oil ameliorates cognition and neuropathology of the 3xTg mice: role of autophagy Annals of Clinical and Translational Neurology (2017) doi: 10.1002/acn3.431
Just a couple of months later another paper by same authors Lauretti and Pratico followed, where canola oil was proven to be neurotoxic, a pure poison really. Important difference is also that the canola oil feed led to no changes in autophagy or inflammation in mice, or so the authors interpreted their data. This was the canola paper:
Elisabetta Lauretti, Domenico Praticò Effect of canola oil consumption on memory, synapse and neuropathology in the triple transgenic mouse model of Alzheimer’s disease Scientific Reports (2017) doi: 10.1038/s41598-017-17373-3
Also there, there was a press release:
“Even though canola oil is a vegetable oil, we need to be careful before we say that it is healthy,” Dr. Praticò said. “Based on the evidence from this study, canola oil should not be thought of as being equivalent to oils with proven health benefits.”
But something was wrong with these two papers, as Cheshire noticed and commented on PubPeer:

The issue here is that it seems the control mice are same, because the experiment with canola and olive oils was done simultaneously, with same control mice. This is why the authors reused controls (something they should have disclosed in the paper), but the really puzzling bit is why they replaced the PS1 blot. The canola oil (CO) paper says: “Compared with controls, no changes in the levels of all proteins investigated were observed when the canola oil group was compared with control mice (i.e., APP, BACE1, ADAM10, Nicastrin, Pen 2, PS1, APH1) (Fig. 2D,E)“. But the olive oil (EVOO) paper says: “No changes were observed in the levels of APP and three of the four components of γ‐secretase (i.e., APH1, nicastrin, and Pen2) when the two groups were compared (Fig. 2E and F)“, implying there were changes in the fourth component, PS1. But then again, a closer examination of the western blot signal suggests the authors might have misinterpreted their findings of “no changes” completely.
It gets better.
The key difference was induction vs non-induction of autophagy, as authors discussed: “Finally, since we previously reported that olive oil is an activator in vivo of the autophagic machinery15, we also investigated whether or not this was the case in the mice receiving the canola oil-rich diet. Assessment of several well-established markers of autophagy activation in the brain of the two groups of mice did not show any significant differences, suggesting that canola oil does not influence this system38“
Turned out, the key figures, on brain inflammation (Figures 4D/E) and on autophagy (Figures 5C) are the only ones where the control blots are different between the two papers. Cheshire kindly posted this analysis of mine on PubPeer, as I am not really welcome there:
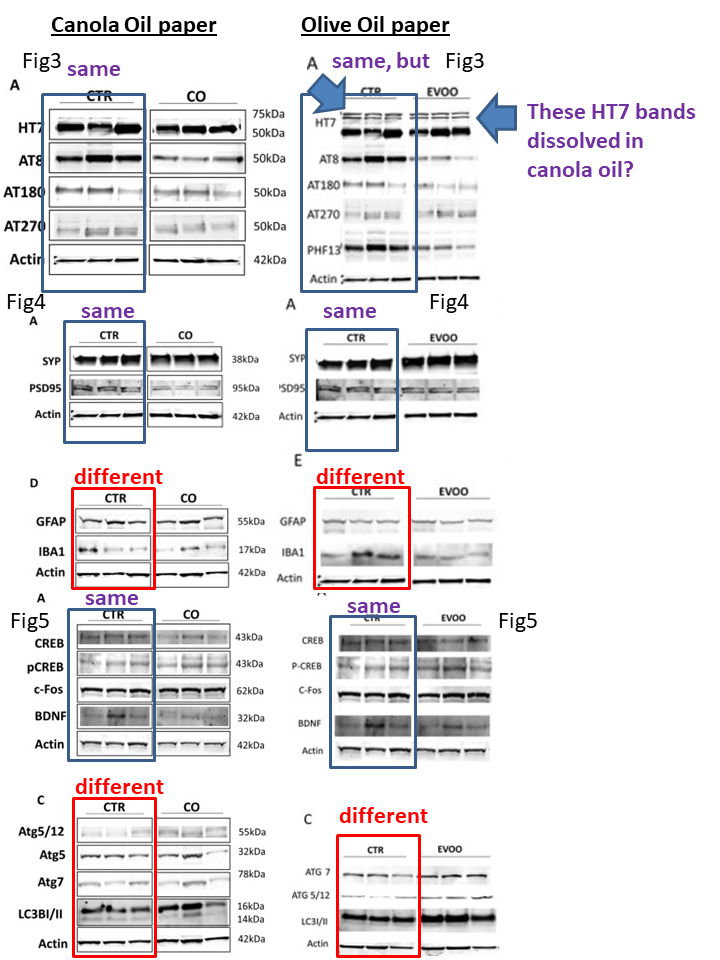
If you swap controls in Figure 5, you will notice that this claim by authors about the benefits of olive oils turns to bullshit:
“As shown in Figure 5C, ATG5 and ATG7 immunoreactivity was significantly stronger in EVOO‐treated mice compared to controls suggesting induction of autophagy“.
Same goes for inflammation reduction:
“In addition, we observed that compared with controls, mice receiving the EVOO‐rich diet had a significant reduction in the steady state levels of IBA1, a marker of microglia activation, which was also confirmed by immunohistochemistry (Fig. 4 E and F)“.
Not if you swap controls with the canola oil paper, then IBA1 basic levels in control mice are rather small and don’t seem to decrease as the olive oil paper claims. More bullshit then.
In any case, a diligent reader might notice that the CO and EVOO panels in the two papers show very similar results (not digitally identical as usually flagged on PubPeer, but with same scientific message), something peer reviewers should have spotted when comparing these papers. But those peer reviewers also for some reason didn’t mind something as obvious as the control and the oil-fed mice not being on a continuous gel.
Basically, the real scientific message is that feeding mice with oil, any oil, instead of standard chow might slightly change gene expression, while the authors claimed olive oil was good and canola oil was bad. In this regard, a comment by D Majerowicz on the now defunct PubMed Commons, preserved on PubPeer:
“In my opinion, a significant control group is missing in this study. The control group should not be a chow diet fed, but a chow diet supplement with olive oil, soy oil or any other oil of this kind. This control would exclude the possibility that the obtained results were caused by a high-fat diet and not by the canola oil. In the current study design, it is impossible to say if the significant effect came from the overall increase in dietary fat or from the specific fatty acid composition of the canola oil.“
A similar criticism was raised by James Roede in a letter to editor. Never mind, in 2019 Pratico followed up on his olive oil research with another paper, Lauretti et al Aging Cell 2019. The work was founded by a charity (North Star Foundation) which seeks to support hospitals serving poor people, by a direct “chair” grant to Pratico. It was celebrated in Forbes (as was earlier oil research by Pratico), where the Italian doctor announced that olive oil “can protect the brain against different forms of dementia“.
The Temple of Photoshop
Pratico is the master of bullshittery, each time he is ready with a new and marketable drug. No wonder that Temple University cherishes him, and the recent $3.8 million governmental grant drives home the point about how science is supposed to be done. Earlier this year, Pratico announced yet another Alzheimer’s drug:
“Our chaperone drug specifically restored levels of a sorting molecule known as VPS35, which helps move proteins out of endosomes, compartments inside cells where proteins are sorted for degradation“.
This was the paper:

Jian-Guo Li, Jin Chiu, Mercy Ramanjulu, Benjamin E. Blass, Domenico Praticò A pharmacological chaperone improves memory by reducing Aβ and tau neuropathology in a mouse model with plaques and tangles Molecular Neurodegeneration (2020) doi: 10.1186/s13024-019-0350-4
The western blots in Figure 3E are not really a piece of research data, but rather a stamp collection by some deceptive maniac.
And guess what. The Y maze mouse behaviour data seems off in that paper, too. Mice of same genetic background acted completely differently when sent to wander in a labyrinth. Sometimes they showed 55% alteration, sometimes 30%, and in the most recent 2020 paper they showed just 10% alteration, as it was flagged in the February 2020 whistleblower report:

There are two explanations for that:
- The mice have conspired against Professor Pratico, enticed by the whistleblowers with bribes of cheese. Very ratty behaviour, after wasting so much expensive olive oil on the ungrateful bastards.
- The alteration values of 3xTg control mice had to match the data of treated mice. If say, the 2020 Mol Neurodegen paper used instead of 10% the number from any of the other two papers (30% or 55%) to compare with the ~30% of treated mice (3xTh/TPT), the result would be the exact opposite of what it was declared to be, and vice versa. The papers would be all unpublishable then, as there would be no difference between treated and control mice in the Y maze assay.
I am sure the Temple University will go for the explanation 1.

PS: I contacted the Katz medical school and Dr Pratico himself for comments, but have received no reply yet.
Update 24.10.2020
But Michele Masucci, Temple University’s Vice-President for Research, replied:
“Dear Mr. Schneider,
Thank you for your email notification. This matter is being evaluated through the appropriate university process.“
Donate!
If you are interested to support my work, you can leave here a small tip of $5. Or several of small tips, just increase the amount as you like (2x=€10; 5x=€25). I need to place my annual order for cold-pressed unfiltered olive oil soon.
5.00 €


Pingback: Espressione di di incredulità - Ocasapiens - Blog - Repubblica.it
Pingback: Antonio Giordano and the Sbarro Pizza Temple – For Better Science
Pingback: mTOR: conclusions not affected? – For Better Science
Pingback: Steven Houser and the Temple of Fraud – For Better Science
Added some more findings today. When reporting to Temple’s IRB, I received this auto-reply:
“The IRB Office is experience extreme staffing issues at the moment. We are monitoring this email address as well as the main phone line. However, please expect delays in responses and reviews. Any “rush job” requests must have a documented, inflexible, and impending deadline.
Prior to asking for assistance with submitting to the IRB (aside from changing the PI or Department Head), please consult our slides on how to submit to the IRB (https://atlas.ocis.temple.edu/research/researchadmin/era/docs/v15/era_irb_quick_guide.pdf). They include screenshots on how to find protocols and consents, start submissions, and respond to Modifications Required to Secure Approval.
I apologize for the inconvenience. “
LikeLike
Pingback: Research misconduct: Theory & Pratico – For Better Science