Giorgio Zauli is Italian clinical haematologist and cancer researcher, who used to be director of Burlo Garofolo Pediatric Institute in Trieste, till in 2015 he was appointed Rector of the respected University of Ferrara. In this position he might soon be investigating his own papers, because given the massive evidence uncovered by the pseudonymous data integrity sleuth Clare Francis and others, Zauli seems to become a second Alfredo Fusco, and given his Rector status, probably just as unshakable. In Italy, science and politics are unhealthily interconnected, as demonstrated by the deadly scandal of Paolo Macchiarini in Florence. In any case, the list of gross irregularities in Zauli’s papers on PubPeer grows daily.
Zauli’s impressive publishing record consists of almost 200 first- and last-author papers in prestigious journals and his h-index bears the impressive size of 57. Some of this academic output, largely co-authored by his close associate and head of his department in Ferrara, Paola Secchiero (h-index 41), was achieved by their rather questionable attitude to data integrity. Of course we have the usual: western blots with duplicated gel lanes and bands re-appearing in several figures and papers, standing in for different experiments and even different patients. This article however will focus on Zauli’s flow cytometry studies, and how such data in his publications apparently got manipulated in a way which is supposed to provide deniability should the authors ever be caught.
Flow cytometry or FACS (fluorescence-activated cell sorting) is a technology to quantitatively measure specific signal on the level of individual cells. Basically, live (or fixated) cells in suspension are loaded into the FACS device where they flow through a thin tube while being shot at with lasers. Unlike microscopy, FACS doesn’t tell you where the fluorescent signal on the cell is exactly located, but it provides a measurement of relative signal strength and analyses thousands of cells in a matter of a minute. The resulting scatter plots show all these thousands of cells as individual dots between two axes of two distinct fluorescence measurements (also a one-axis plot is possible, where individual cell dots form a histogram).
Various cellular signals can be detected by FACS, this high-throughput technology is in particular popular in haematology, where cells are already present in high numbers in suspension (e.g., blood). Zauli’s team used it to detect the signal of fluorescent antibody binding to specific proteins on cell surface, or to measure proliferation of normal or cancerous cells by staining their DNA. Shown as figures were what authors called “representative” FACS measurements, but occasionally those were actually same old files re-presented in as new, unrelated experiments. Sometimes plots were just copy-pasted, and sometimes the original FACS files must have been sneakily re-gated to make the resulting plots appear sufficiently different.
Let us start with a simple example of Zauli’s handling of flow cytometry data: the Wandering FACS of Ferrara, courtesy of Clare Francis


All these figures above show cell death assays, where DNA dye propidium iodide (PI) and fluorescent Annexin V protein probe are used to detect apoptosis-specific changes on the outer cell membrane and fragmented DNA of dead cells, respectively. Colour-coding shows which plot is duplicated where, standing in for different cell types in utterly unrelated experiments, their only common aspect being the general Annexin V/PI FACS technique.
This happened across four papers, all featuring Zauli as key author: Secciero et al J Leucocyte Biology 2007, Secciero et al Neoplasia 2007, Secciero et al Circulation Research 2007, Zauli et al Bone & Mineral Research 2007. On the latter paper, Paola Secchiero is last author, all of which definitely helped her to full professorship at Zauli’s University of Ferrara and in 2015, the year of Zauli’s appointment as rector, she became director of the Department of Morphology, Surgery and Experimental Medicine, where Zauli’s own lab is.
The oldest Zauli paper flagged on PubPeer, which happened already one year ago, is Gibellini et al J Immunology 1998. That was before flow cytometry became a half-way affordable technology for most labs and even before the Golden Era of Photoshop. Shown below is an electrophoretic mobility shift assay (EMSA) to measure protein binding to DNA, and one wonders which tricks Zauli’s team had to use in 1998 to get a gel lane incidentally duplicated. Probably the good old paper-scissors-glue game, where you make a collage of photographs of same gel and then photograph your collage again, to publish the final figure of an allegedly monolithic, unadulterated gel; a technique also applied by German scientists in 2000, as told in this article.

Zauli however had one of those (at the time prohibitively expensive) FACS devices, and he put it to good use, to make papers in the elite journal of the haematology field, Blood, published by The American Society of Hematology. The paper Zauli et al Blood 1999 has flow cytometry data, only occasionally of a duplicated kind. The histograms below show FACS data of various cell types stained for surface protein CD95, two different measurements are shown in each plot. The grey-shadowed negative controls (cells not stained for CD95) are reused, only that the cell lines which the histograms stand for, are clearly different. Basically, this suggests Zauli’s team failed to make proper negative controls, without which such FACS results are utterly useless.

The journal Blood was known in the past to largely ignore reports of data manipulation. I myself had this experience in 2015 when I forwarded to the academic Editor-in-Chief Bob Löwenberg original evidence in a relatively fresh case, where French authors manipulated Real-Time-PCR data to accommodate reviewer concerns (evidence on Figshare here, and the article here, pages 24-27). The journal acknowledged my emails, did nothing at all since, and eventually stopped replying. Now, Blood beats the big drum of research integrity, like this Twitter thread from the 2018 Council of Science Editors conference shows:
As it seems, Blood is only concerned about research integrity in papers they have not published yet. Afterwards: anything goes, the deed is done and no one cares. One can safely assume the journal will ignore this duplicated FACS data from Secciero et al Blood 2008, and this is where Zauli et al did something much more creative than just copy-pasting data sets.

The experiments in Zauli et al Bone & Mineral Research 2007 and Secchiero et al Blood 2008, show two clearly distinct cell types, peripheral blood mononuclear cells (PBMCs) in former and human umbilical vein endothelial cells (HUVECs) in the latter. Two plots are marked with red boxes, both controls showing control cells not treated with Nutlin-3 or other inhibitors. For the purpose of studying cell proliferation, DNA was labelled with a traceable bromo-nucleotide BrdU, thus visualising the DNA replication rate. The two plots in the two papers are similar, but not same. Rector Zauli and Head of Department Secchiero, should they be ever asked to investigate themselves (this is the pleasant advantage of being the top boss of your research institution, as this British case shows), would probably use the partial dissimilarity as evidence that those plots show two clearly different measurements.
Not so fast: these two plots do show the same measurement after all. A FACS device saves each sample measurement as a digital file with its own meta-data, and those files are what any investigation should be asking for first. There are two kinds of settings: first, those made by scientists during the measurement when setting up the device and adjusting negative controls: those are recorded in the file and cannot be changed afterwards. Neither should these device settings be modified during a flow cytometry session between samples of the same experiment, though two scientists admitted to have done just that to get the result they needed to publish a paper in Nature (here).
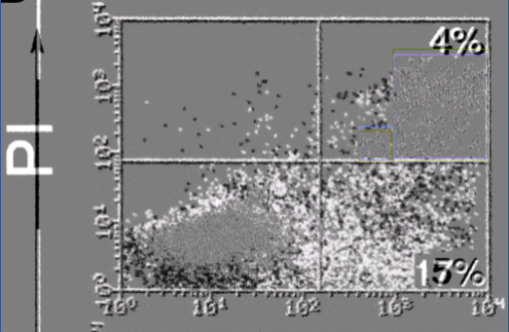
Second kind of flow cytometry settings are those made during data evaluation. The raw files saved by the FACS device are plotted as histograms or scatter graphs like the case at hand. This is where scientists set gates again, by selecting which cells will enter the analysis and which will not. Also here one should apply exactly same gates between samples of the same experiment, or the results become void and useless. Every time one changes the gates ever so slightly, the final histogram or scatter plot will look slightly different. This is what probably happened in the experiment shown above: same FACS file (we cannot know what kind of cells that originally was) was gated slightly differently. The bulk of cells remains the same, while others populations look different. This is nicely visualised by the PubPeer user Hoya Camphorifolia in this specific case here:

Identical dots (cells) cancel each other out, the black and white dots are the ones different between the two gated plots. These are quite likely the same FACS measurement re-gated and re-plotted. Re-presentative, as Zauli and Secciero would say.
Here is a similar case, from same Secchiero et al Neoplasia 2007 and Secchiero et al Circulation Research 2007 papers mentioned before. We see two kinds of FACS data duplications (again different cell types are used in the two papers, the only common feature being the Annexin V/PI staining technique): duplicated identical scatter plots and duplicated re-gated scatter plots, inside each paper and between them.

The striking thing in the top panel (Neoplasia 2007 paper): the first 3 plots are apparently the same FACS sample, re-gated. The little colour boxes indicate the common elements, unique groups of individual cells which cannot be same by chance. Additionally, same new plots of a plot were re-used again, in the Circulation Research 2007 paper, in a different context. Because of this, we cannot know which cells those originally were, it can be HL-60 or SKW or HUVEC or PBMC cells, or something else altogether. Here again Hoya Camphorifolia visualises on PubPeer the overlap of the bulk cell population in those 3 plots:

There are even weirder cases. Here, in Secchiero et al Am J Pathology 2005, a small part of the two plots overlaps exactly, while the rest is very different. A weird case of a crazy and rather improbable coincidence? Or maybe someone just got tired of playing with settings and simply photoshopped part of one FACS plot on top of another? Only the authors can explain.
After this discourse into flow cytometry, my regular readers might be demanding more of those rigged Western blots they are used to see on my site. Here you go then. So much more for the new heroes of research integrity at the journal Blood to ignore. Update: the journal seems to care after all,see this tweet:
Let’s for example follow the fate of the yellow-boxed gel loading control from the paper Secchiero et al Blood 2006, standing in for patients 2 and 14, who suffered from B-cell chronic lymphocytic leukemia (B-CLL). It returns in Secchiero et al Neoplasia 2007, and now it shows the blood samples of a patient 4, suffering from acute myeloid leukemia (AML), treated with Nutlin-3. One can only hope the good doctor Zauli discriminates better between his patients when actually treating them. Especially because in 2009, that same western blot loading control of primary patient blood made another appearance as not one, but two different leukaemia cell lines BJAB and SKW.
 And this is how Professors Zauli and Secchiero made their impressive Italian research careers to the very top of their University of Ferrara. The Rector and the Head of Department can now investigate each other.
And this is how Professors Zauli and Secchiero made their impressive Italian research careers to the very top of their University of Ferrara. The Rector and the Head of Department can now investigate each other.
Update 21.05.2018. I reported the evidence to the Ethics Commission of the University of Ferrara. Also, a first-hand source informed me that Rector Zauli announced to sue me for libel. This is today’s message on both points from the university’s official, Cinzia Mancini:
“the Ethics Committee will first examine the admissibility of your request for opinion at the next meeting, convened for Friday, 1 June 2018.
Regarding the alleged complaint, the Commission has no information on this matter”.
And then on 23.05.2018 Zauli reported me to Italian state prosecutor and threatened a lawsuit, in an official letter from his University of Ferrara.

Donate!
If you are interested to support my work, you can leave here a small tip of $5. Or several of small tips, just increase the amount as you like (2x=€10; 5x=€25). Your generous patronage of my journalism will be most appreciated!
€5.00



Pingback: Giorgio Zauli: Nazi comparisons, legal threats and totalitarian rule in Ferarra – For Better Science
Pingback: Ferrara protects Rector Zauli from “prejudiced” media and unqualified public – For Better Science
Pingback: Cinque cose (+1) sul caso Zauli – Daniele Oppo
Pingback: The Crooks of CRUK – For Better Science
Pingback: The Teachings of Chairman Cao – For Better Science