The travesty around Cassava Sciences and their alleged miracle drug for Alzheimer’s, Simufilam, waits to be dramatised for screen or stage, a real-life parody on US stock-market capitalism.
It’s better than Theranos even. Apparently even more criminal.
What obviously happened here is that a gang of crooks was that bold to shamelessly fabricate the preclinical lab data and the clinical trial results, engaging some unbelievably crooked academic and trial site partners-in-crime, that the stock-market and the most respected authorities, NIH, FDA and a learned society, swallowed it hook, like and sinker. And they are reluctant to let go.
For investors, Cassava became a religion of financial deliverance: a miracle Alzheimer’s drug, out of nowhere. The more outrageous Cassava lies of clinical success became, the higher its stock rose. And rose, and rose, the company was last valued at $5 Billion. Nobody wondered, huh, all these results are way to good to be true and make scientifically zero sense. Until some inquisitive scientists decided to play the stock market as well.

This is probably the world premiere of data integrity sleuths seeking to earn money with manipulating the stock market by exposing research fraud. At least two sets of them are now short-trading Cassava stocks, betting on the stock value to drop as they reveal lies and fabrications this company submitted in scientific journals and FDA. Others are piling on.
Maybe it is a way forward to deal with research fraud in biotech. Or maybe not. Cassava is indeed too fake, their owner Remi Barbier, his wife Lindsay Burns, and their partners like Professor Hoau-Yan Wang, are all just too cartoonishly crooked and scientifically illiterate to be anywhere near credible as characters in a screenplay about the abysses of biotech entrepreneurship. And yet there are still hordes of investors fanatically defending Cassava, also they and their nasty online harassment of critics would really stretch credibility in a movie dramatisation. Real life however is much stranger and more riveting than any fiction.
Citizen’s Petition
It all started in August 2021 with a “Citizen’s Petition” by the law firm Labaton Sucharow to the FDA, which FDA published on its website, and which I covered extensively in my earlier article.
The petition, submitted by someone holding short-sell stock options, was titled:
Statement of Concern Regarding the Accuracy and Integrity of Clinical and Preclinical Data Supporting the Ongoing Clinical Evaluation of Compound PTI-125, Also Known As Simufilam re Citizen Petition from Labaton Sucharow
It presented evidence of data forgeries in Cassava’s preclinical research in the City University of New York (CUNY) lab of their board member Wang. Also some irregularities in Cassava’s clinical trial data were discussed.
Someone on Twitter brought this FDA submission to the attention of the image integrity sleuth Elisabeth Bik, who then had a look and found even more forgeries. Not just fake western blots from Wang’s lab, co-authored by Burns. Bik also found evidence of fabricated clinical trial results.
CUNY opened a research misconduct investigation against Wang. The Wall Street Journal reported in November 2021 that Cassava, “the sixth-best performing U.S. stock this year“, once valued at $5 Billion, was under investigation by the Securities and Exchange Commission (SEC), the US government’s stockmarket watchdog. Cassava was also said to be under investigation by National Institutes of Health (NIH), who may want to recover their $20 million grant money.
And from the WSJ article, we learned who submitted that FDA letter:
“The petition’s authors are two physicians who said they came to doubt Cassava’s research and have shorted its stock, betting the share price would fall once investors recognized the problems they found, they said. David Bredt, a biotech entrepreneur and former neuroscience research chief at Johnson & Johnson and Eli Lilly & Co., and Geoffrey Pitt, a cardiologist and professor at Weill Cornell Medicine, wrote that Cassava’s research, published in several different scientific journals, include images of experiments that appear to have been manipulated using software such as Photoshop.”
Again, for an explainer on this report by Bredt and Pitt, and on Elisabeth Bik’s findings, read my previous article; and for the first hand source, visit Bik’s website (here and here).
Invest in Biotech
Stock short-selling is basically betting on a stock price to drop. You “borrow” a certain number of a company’s stock from a trader (for a fee), and then sell it. You later on buy this same number of stocks again so you can return what you borrowed. If the stock price fell in between, you earned money. If it rose, you lost.
Basically, if you know a stock-market-listed biotech company is built on fraud it is a clever strategy to get rich by betting on its stock decline. Especially if you have evidence of fraud which you can release to influence the market. Try with Sanofi 😉
It is however a risk. What if nobody believes your evidence? What if the company simply sues you for libel, that will be expensive. And what if nobody simply cares if the business model is phony trash as long as it attracts gullible buyers? After all, the Harvard professor David Sinclair made off with $750 Million from GlaxoSmithKline for his Sirtis scam, and he is now running another profitable quack business, Elysium Health.
This is why I would never be able to advise anyone on short-term biotech investments, because in the capitalist stock-market gamble it doesn’t matter if the product is medically promising or not. If you aim to earn money short-term, you invest on other criteria than scientific trustworthiness. It’s very different for long-term investments, where a business model built on bullshit can hardly lead anywhere. Again, GSK lost much more than the $750 Million they paid Sinclair by trying to make his resveratrol drugs to work, before giving up and dissolving his Sirtis. And how is Unity Biotech with its senolytics doing? Not that I pity Jeff Bezos’ and Peter Thiel’s investments.
But then again, Sirtis or Unity were never stock-market traded like Cassava. And apparently, when a mom-and-pop business is suddenly valued at $5 billion, shrewd stock-market gamblers will check for weaknesses to cash in. Thus, another set of sleuths followed Bredt & Pitt’s example and decided to earn some money by exposing Cassava fraud. Really, there is enough to expose, for everyone. A bottomless never-ending treasure trove of Cassava fraud.
The team comprised of 4 men, Enea Milioris (a biotech trader), Adrian Heilbut (a bioinformatician), Jesse Brodkin (a “small business owner”), and Patrick Markey (a “Psychologist, Investor“).
The four men published their findings as a letter to FDA and in 3 PowerPoint presentations on this website: www.cassavafraud.com
They also openly declare:
“The authors of this presentation and the associated letter to FDA hold stock and options positions that may benefit from a decline in Cassava Sciences’ stock price.”
Here is their 23-page long letter to FDA:
Addiction of Fraud
The CassavaFraud sleuths openly say there is absolutely no science behind Cassava’s Alzheimer’s Disease (AD) product Simufilam (whatever it is):
“The foundation of Simufilam’s action is biologically implausible. According to Cassava:
● a key structural protein Filamin A (FLNA) is found almost entirely in a misfolded state in AD patients’ neural AND blood cells – but not in healthy individuals
FLNA has been studied for over 35 years and acts as a key scaffold for a wide range of signaling proteins. Yet Cassava alone have reported its critical role in AD”
No science, just fiction. And it was not the first time they tried this scam.

What changed, is the company’s name and the market they target. Before Cassava’s focus on Alzheimer’s, Pain Therapeutics tried to cash-in on the US opioid epidemic and to get their own addictive opioid drug licenced. How very suitable for these people’s morals.
“Pain Therapeutics to Cassava
● Pain Therapeutics (PTIE): Founded in 1998 by Remi Barbier, with Dr Friedmann
● Early preclinical research in analgesia, including studies of low-dose Naloxone
● In-licensed Remoxy, a supposedly tamper-resistant version of Oxycodone
● Remoxy was first rejected by FDA in 2008
● Nevertheless, Remi persisted.. and Remoxy was rejected again and again
● Remoxy was rejected for the last time in 2018, leading to ‘disoriented’ Remi’s famous diatribe against the ‘shambolic regulations’ at the FDA
● In 2017 Cassava begins a pivot to Alzheimer’s Disease (AD) based on Wang’s 2008 research with Pain Therapeutics and their novel drug: Simufilam
● The rebranded company miraculously hits milestone after milestone in a record of unprecedented clinical success in AD – both in the treatment and diagnosis of the disease”
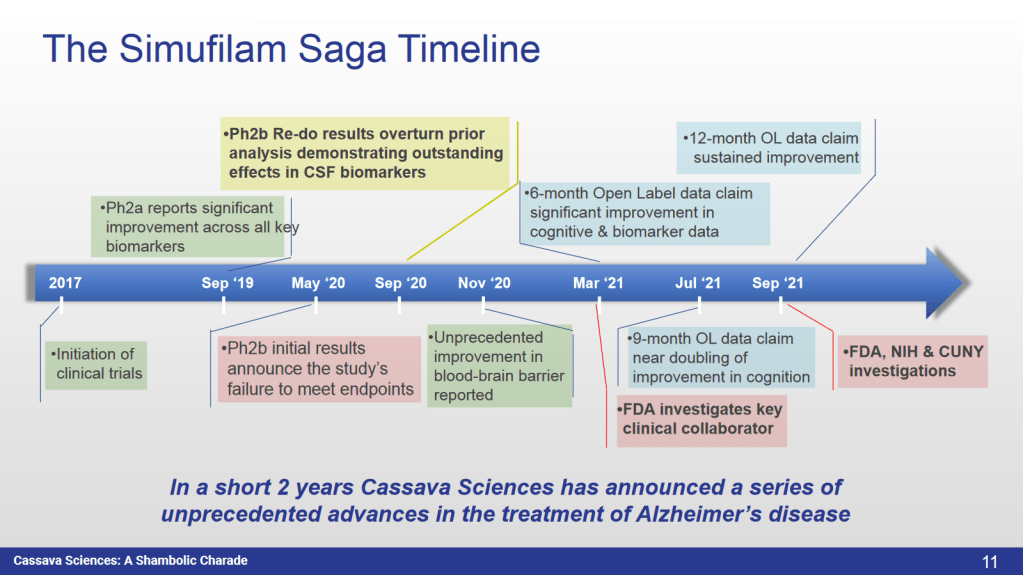
This time, the company is renamed to Cassava (after Barbier’s and Burns’ home address), and the drug is for Alzheimer’s, a huge global market where no therapy exists and where even the drugs which don’t really work (like Biogen’s Aduhelm) get approved because this is how desperate everyone is.
Alzheimer’s Bonanza
Of course Cassava is not the only shady company gambling on this desperate situation. Remember senolytics? Dr Miranda Orr of Wake Forest School of Medicine decided that Alzheimer’s is caused by lack of cell division in the brain. Sigh, yes, I know. Hence senolytics, to kill these presumably senescent cells with a drug decreed to be “cyclin-dependent kinase inhibitor 2D (CDKN2D/p19)“. Like Cassava, Dr Orr developed her own assay to discover things pedestrian scientists won’t see:
“They developed a senescence eigengene approach to identify rare, senescent, cells within large, diverse populations of postmortem human brain cells. Eigengenes, the team noted, are useful when no single gene reliably captures a phenotype, like senescence.
By profiling tens of thousands of cells from the postmortem brains of people who had died with AD, the team found that approximately 2% of cells were senescent cells.”
Yes, it is all very silly, but because Dr Orr paid to publish it in Nature Aging and patented her therapy, a clinical trial (NCT04685590) is set up, sponsored by Wake Forest and in collaboration with UT San Antonio.
The drugs used in this phase 2 trial by Dr Orr with 48 Alzheimer’s patients are Dasatinib (a chemotherapeutic) and Quercetin (fresh fruit ingredient), incidentally the same magic senolytic drugs which cure COVID-19, according to Nature and Charite Berlin!
Pray for Dr Orr’s human guinea pigs, dasatinib has very heavy side effects, this is why sane doctors only give it to people with leukaemia.
If you seek yet another shady Alzheimer’s biotech start-up to short-sell, here is Alzamend. They licenced “a mutant-peptide sensitized cell as a cell-based therapeutic vaccine that reduces beta-amyloid plaque” from University of Florida, which was recently found, both by sleuths and by the university, to be fraudulent and “not scientifically defensible“. The relevant paper Habib et al 2017 was retracted.
If you make money betting on Alzamend stock to crash, send some Cheshire’s way.
Also related to the case at hand: Athira Pharma was exposed for similar problems as Cassava, its once-celebrated star CEO Leen Kawas was caught with manipulated data from her past PhD work with Athira’s founders, two University of Washington professors.
The company swiftly dismissed Kawas and insisted in a press release that her research had nothing to do with the Alzheimer’s drug:
“The Company and Dr. Kawas agreed it is in Athira’s best interest to enter this critical next chapter under new leadership. Dr. Kawas’s actions at Washington State University took place many years ago and did not involve ATH-1017, Athira’s lead development candidate,“
That last bit is actually untrue. ATH-1017 is the physiological precursor of the same drug “dihexa” which Kawas faked her PhD research with.
Dr Wang’s magic
Unlike privately-funded Athira, the stock-market traded Cassava went on the offensive. After all, Barbier can hardly dismiss his wife to gain credibility, and Wang is also, in a way, almost part of the family. The company simply continues to make up results and issue grand promises. After all, it worked like a dream so far.
The CassavaFraud report sums up:
“Too good to be true?
● Cassava has claimed a series of significant and unprecedented clinical “milestones” in AD
● In nearly every patient and after only 28 days of treatment with Simufilam, Cassava claimed:
○ significant reduction biomarkers of neurodegeneration & neuroinflammation
○ significant increases biomarkers of blood-brain barrier (BBB) integrity
○ significant reduction in biomarkers of Alzheimer’s in the blood
○ improvement in patient’s cognition
● In follow-up, open label studies, Cassava claims the drug showed sustained improvement or stabilization in cognitive function for up to 12 months
● Nearly every reported outcome is a world-first in AD treatment
● To top it all off, Cassava claims to have successfully developed a blood-based diagnostic that detects AD prior to any symptoms with >98% accuracy”

And the CUNY professor Wang plays a key role. Without him magically turning sh*t to gold, there would simply be no Simufilam and no Cassava.
“Dr Wang’s lab alone analysed the biomarker data…
● Dr Wang and his laboratory were ultimately entrusted to analyze nearly ALL the clinical samples of the Simufilam program
● On review of the reported Phase 2b data; 7 of 9 CSF biomarker readings are either:
○ entirely inconsistent with scientific literature
○ in ranges incompatible with human biology
○ compatible only with alternative analytical methods then those reportedly employed”

It wasn’t just the western blots of the preclinical research which Wang faked. When clinical trial data showed no effect for Simufilam, Wang took over and the magic happened:
“The story behind the data
● In our view, the failure of the original analysis was choreographed to justify the analysis of samples by Dr Wang’s lab who could produce desirable outcomes
● In signature fashion, the fabrication of results becomes evident upon basic scrutiny by experts
● The attempted simulation of ELISA results based on data from Luminex assays is, like Dr. Wang’s photoshopped westerns, comical and grave at the same time
● The choice of WB method to measure albumin ratio is likely an attempt to publish “film evidence” in support of the unprecedented finding of BBB integrity improvement
● Cassava Sciences have inexplicably entrusted one of their own instead of recruiting accredited providers per standard industry practice
● To inquiries on the puzzling data reported, the company’s CSO Dr Burns responds with outrageous excuses and no concern for quality control as expected of a study sponsor”
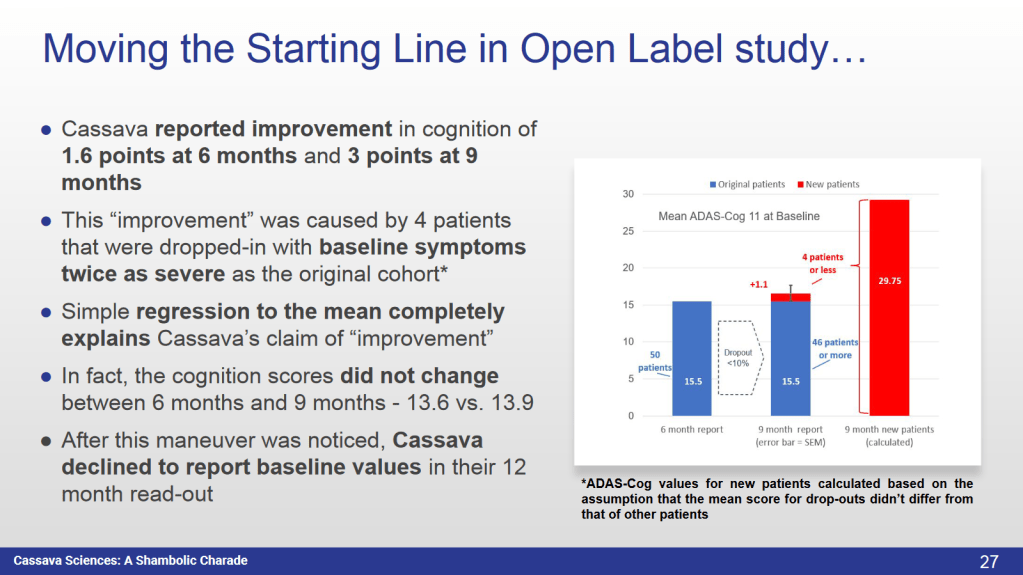
● Each assay had a customized mix of exclusion criteria applied
● As much as 40% of data was creatively removed”
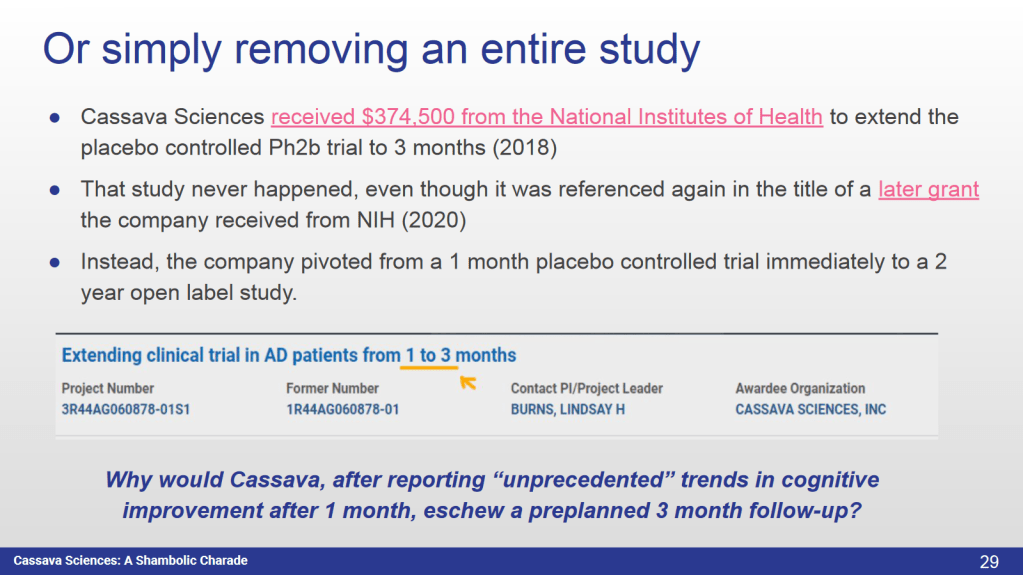
An entire NIH-funded 3-month placebo controlled study either never happened or ended in trash, because someone didn’t like the results. And what was left, was falsified.
“Questions on Cognitive Data
● Were Ph2b post-hoc outlier criteria designed to mislead?
● Is Cassava manipulating drop-out replacements for the Open Label study to obscure true effects?
● Are the patients recruited in the trial confirmed mild-to-moderate AD?
● Why were 12 month baseline values from the Open Label study not reported?
● Why has Cassava silently eliminated a preplanned confirmatory and NIH funded 3 month placebo controlled study of the cognitive effects they claim?”
The CassavaFraud report concludes that all Simufilam data was negative, and it was Cassava’s managers and Wang who falsified it to look as if the drug worked:
“Our version of events…
● Cassava Sciences fabricated the failure of sample analysis by an external, accredited lab and avoided the reporting of clinical endpoints (IL-1β) to main
● Closer inspection of the biomarker data generated by Dr Wang show clear evidence of fabrication in an effort to produce favorable readings
● In an effort to manipulate those Phase 2 study outcomes which were out of Dr Wang’s reach (cognition and plasma tests), Cassava Sciences intentionally used
Questionable Research practices* such as patient cherry picking and arbitrary outlier definition in order to obtain favorable results in patients’ cognition data
● The planned blinded 3-month extension study was dropped since blinded cognition data would not have been easy to selectively report (compare, e.g., with non-significant findings in cognition data of blinded Ph2b study)”
Now, it wasn’t just Wang who helped the Cassava managers fake the clinical data. Other investors did their research also, and found more outsourced fraud.
Born-again crack stripper
As I reported before, Cassava’s main clinical trial site partner is IMIC Research, Inc, located in Palmetto Bay, Florida. And what a sh*thouse this is, pardon my French.
First of all, the principal investigator at IMIC for the Cassava trial, Evelyn Lopez-Brignoni, was sanctioned by FDA earlier this year. Here is the Warning Letter:
“You failed to ensure that the investigation was conducted according to the investigational plan [21 CFR 312.60].
As a clinical investigator, you are required to ensure that your clinical studies are conducted in accordance with the investigational plan. The investigational plan for Protocols (b)(4) and (b)(4) required subjects to take (b)(4) of study medication (b)(4), administered as a (b)(4), to demonstrate the efficacy (b)(4). You failed to adhere to these requirements.”
IMIC is owned by another charming husband and wife couple, Boris Nikolov and Aimee Cabo, who are mad as two hatters (in pointy white hoods). Check their Twitter feeds (here and here) at your own peril. It’s mostly inane Jesus-worshipping memes with heavy white-supremacist undertones.
Interspersed with far-right ideology plus rabid antivaxxery and covidiocies. I collected among the recent tweets:
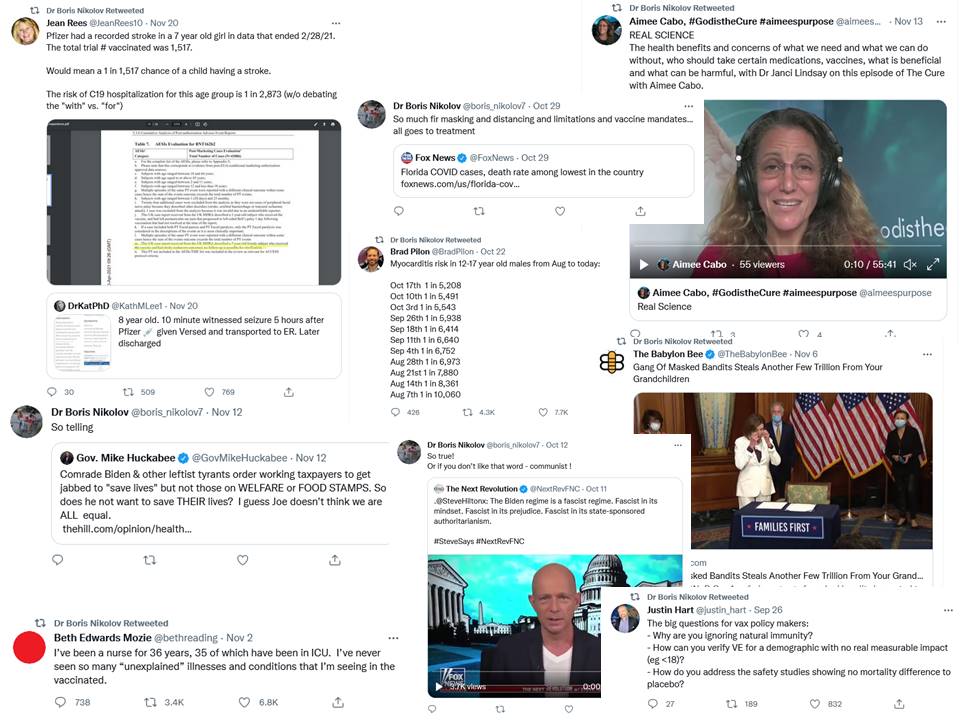
But this is not all. Cabo’s relationship to drugs used to be of a very different kind.
Behold, a long analysis by Quintessential Capital Management (QCM) from November 2021, titled:
Cassava Sciences (SAVA): Game over!
A warning for the US healthcare system
It reveals a lot about Cassava and IMIC:
“Cassava’s prominent clinical research site (whose CEO is coauthor of critical research on Simufilam), IMIC Inc., is co-owned by a former escort, stripper and crack addict with a criminal record for consumption and possession of cocaine.”
But Aimee Cabo is a born-again Christian, so there. Oh, and here is a bizarre story about her in Miami New Times, referenced by QCM.
From QCM; we learn that Cabo and her Bulgarian husband were once both bankrupt, but now became rich thanks to Cassava money. This is how the clinical trials were monitored:
“In one case, we learned that Cassava hired an external monitor that never set foot in the research centers40.
In addition to this, we learned Cassava often reserves itself the right to exclude patients for “anything that in the opinion of the Investigator would preclude participation in a 2-year study 41 ”. Based on our conversations with former employees and with research centers, we understand that such decisions are
frequently taken by Cassava’s management.”
QCM even sent undercover investigators posing as trial candidates to IMIC:
“First, we learned that patients and whoever introduces them to the centers receive sums of money that many consultants have found excessive42 and potentially leading to conflicts in patient selection. In fact, while chatting in the waiting rooms of these centers, we learned that there are large numbers of “professional patients” who go from study to study just to seek these monetary
rewards. Many “patients” during our visit appeared quite normal (cognitively speaking) and were exchanging tips about what to say to the staff in order to get their study money (!)”

As for Nikolov, whom the QCM investigators saw give “a “sales pitch” to join the Simufilam study“, he all but admitted to be a Cassava investor:
“When asked whether he held any stock, he became uncomfortable and ultimately did not answer.”
Quantifying a gel band
Let us go back to the CassavaFraud analysts.
They also had a look at that amazing biomarker assay the company developed, named SavaDx, to measure Alzheimer’s risk and progress. As the promise goes:
“SavaDx can distinguish:
-Healthy elderly from Alzheimer’s patients with 98% accuracy
-Mild impaired (MCI) from Alzheimer’s patients 92% accuracy”
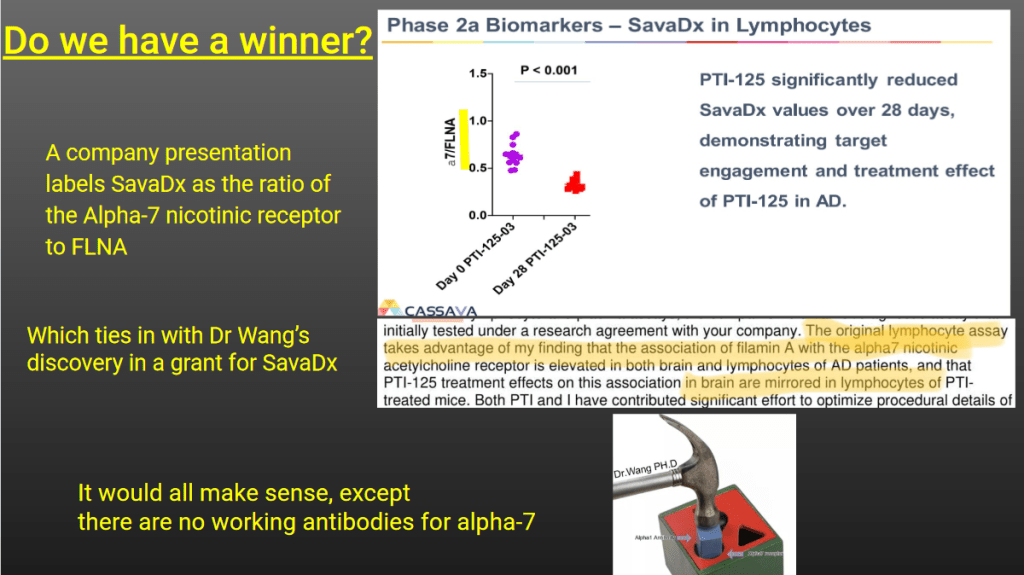
The team submitted a Freedom of Information (FOI) inquiry to SUNY for Wang’s emails. Usually the universities reject such requests outright or fight tooth and nail for months and years, and when needed through courts, just not to release their professors’ emails. Unless the university actually wants to hang their professor out to dry. Then all you have to do is just ask.
“Email between Drs Wang & Xu contains results of a Western Blot analysis of two proteins of 90 & 280 kDa FLNA lysate is used as a positive control, therefore the assay targets the 90 and 280kDa fragments of FLNA
In the analysis we see the 90/280 kDa ratio calculations, plus the 28d vs 0d ratio
Finally, we have the answer to what SavaDx actually is: the ratio of 90/280 kDA FLNA”
You might wonder: which patient tissues did they analyse for Filamin A isoforms by western blot to make it a biomarker for Alzheimer’s? Surely not the brain?
Luckily, not. As Burns admitted, they used blood. How an (allegedly) misfolded protein in the blood would affect the brain, and only the brain alone, where it causes Alzheimer’s, is something so mysterious you yourself will need a brain the size of Wang’s to understand. But wait, there was yet another mystery with this approach.
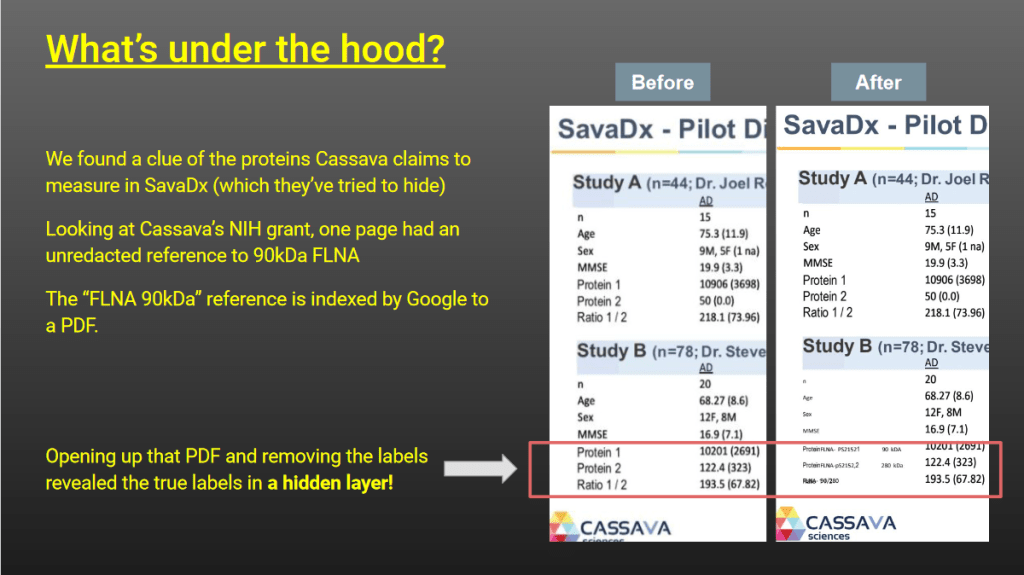
and Dr Wang was included in FOIA’d material”
Now, every failed scientist can calculate the signal ration between two bands, in this case the 280kDa and the 90kDA Filamin A band. Simple. But only a genius like Wang can calculate the ratio of two bands while the gel actually shows just one, the 90kDa band. In fact, there is not even one, but rather none. The real raw data the team obtained by FOI inquiry shows an almost undetectable 90kDa signal at best, and in most cases actually a blank, which miraculously transformed for the very same trial participants into a fat juicy gel band in the Cassava poster, deployed to announce a breakthrough.
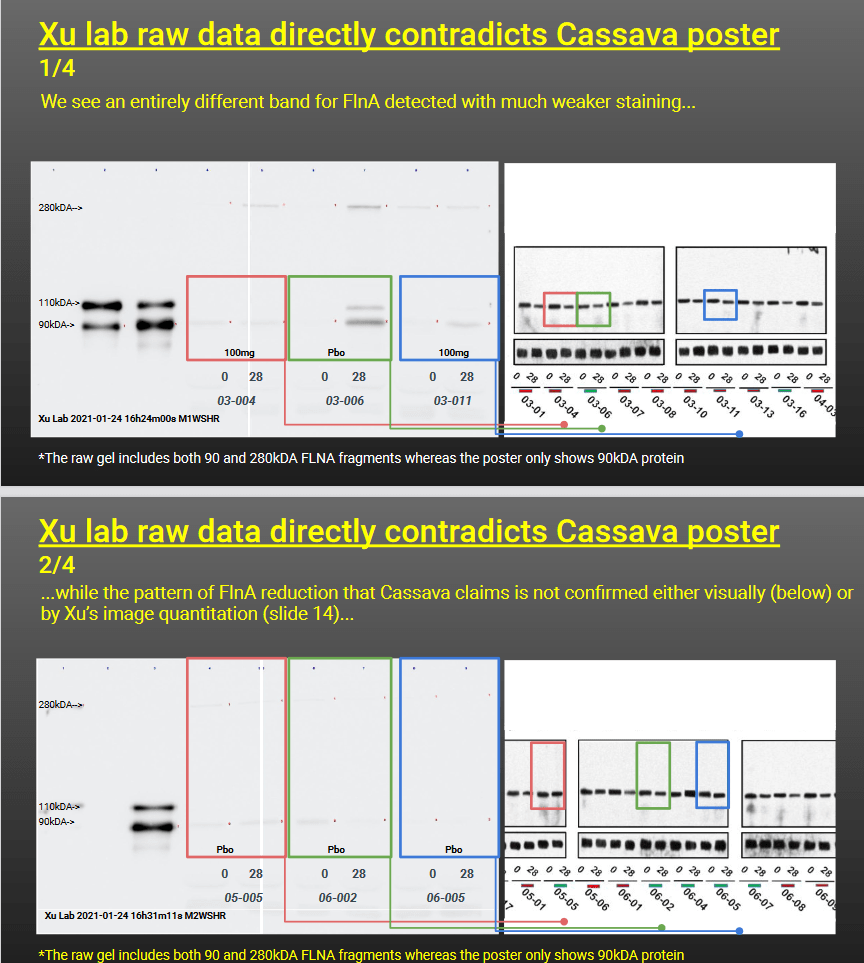
The blind can see
And guess what: Wang always knew which patient received which treatment (if they can be called Alzheimer’s patients, remember that IMIC recruited everyone who wanted to be paid). Wang even knew the names of the trial participants, while Cassava assured everywhere (and still does) that the studies were fully blinded. For example:
“The Xu lab email contains the original Western blot films along with the
quantification results for 12 patients -plus identification numbers”
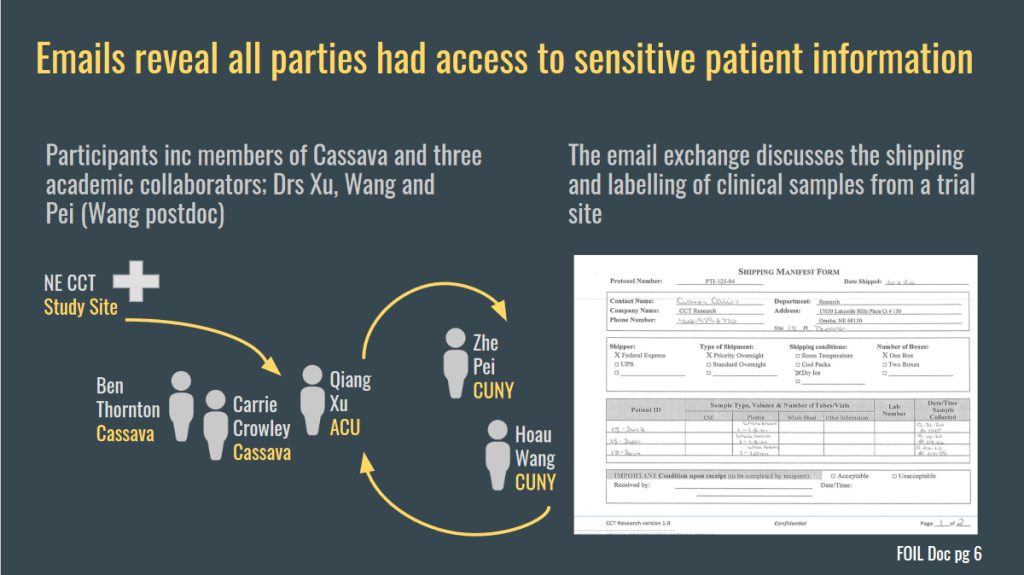
It was not just Wang who had access to the officially blinded and confidential participant information. Everyone did!
“Emails retrieved from a FOIL request to CUNY expose Cassava and the Wang Lab as being unblinded during sample analysis, prior to data presentation and while study is ongoing […]
Hence, whether a patient is ON or OFF the drug is known to the person analyzing samples This could allow Wang* to decide what sample measurements “should be”.
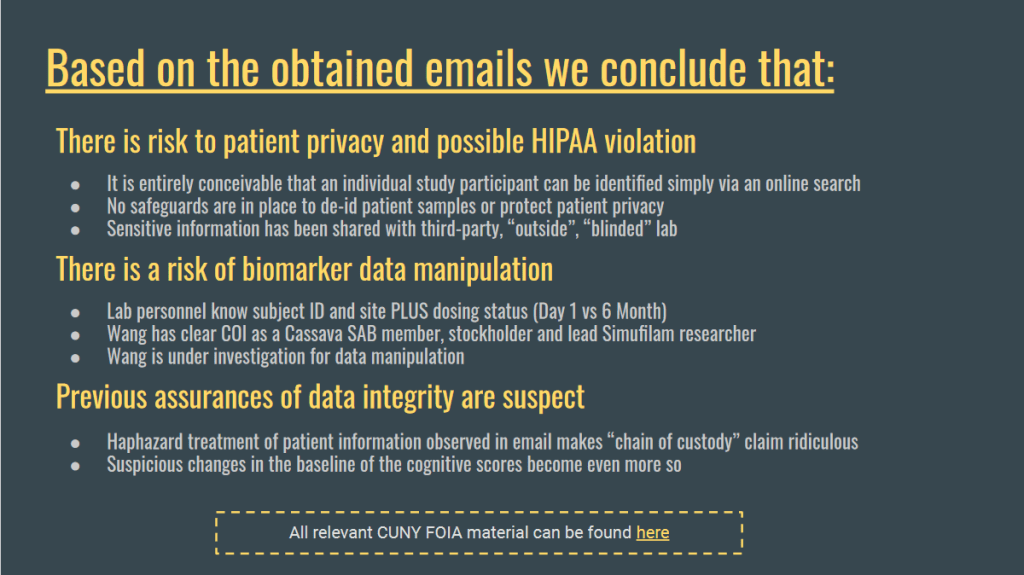
When the need called, Wang simply moved trial participants around, from placebo to treatment cohort or vice versa, or rigged their results, and often just dropped them completely and invented new ones.
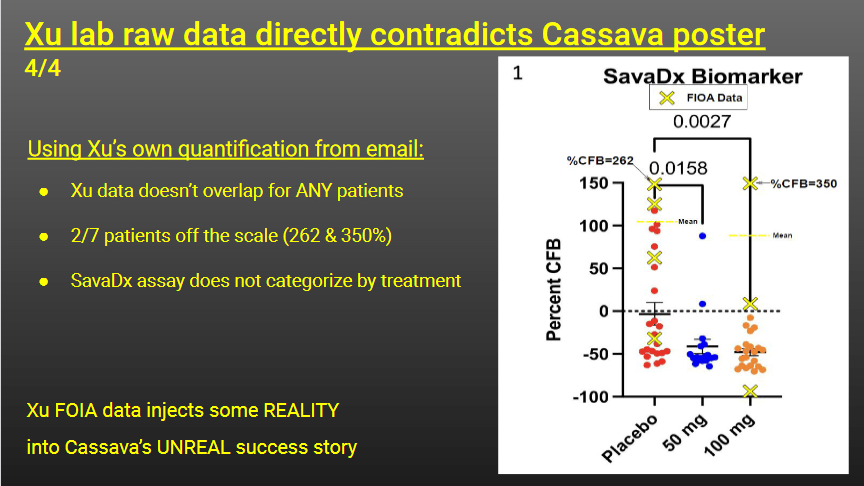
The CassavaFraud sleuths sum up:
“Summary: The State* of SavaDx
● Based on Western Blot quantification = outdated
● Not patented and not a trade secret = $0
● “Validation” clinical trial for 2021 = Cancelled
● Does not show effect of Simufilam treatment = 0% accurate
● Discovered emails suggest numbers totally fabricated = Fraud?”
We are also told:
“*Stay tuned, more FIOA emails by Christmas!”
Concerned Scientists
Now, surely you will think the academic neuroscience community is shocked, shocked, seeing gambling going on here, probably writing letters of their own to FDA, demanding an urgent crackdown on Cassava fraud?
Well, sometimes even I am surprised by the callousness and crookedness of academia. You won’t believe what the world’s biggest academic community of neuroscientists and neurologists, the venerable Society for Neuroscience (SfN), did.
Their flagship Journal of Neuroscience issued on 4 November 2021 a joint press release with Cassava to defend research fraud and to smear Elisabeth Bik. Here it is, regarding the disastrous paper paper Wang et al J Neuroscience 2012:
“The Journal of Neuroscience authorized Cassava Sciences to share a statement on this matter, reprinted in full below:
“The Journal of Neuroscience follows COPE [ C ommittee o n P ublication E thics] guidelines and takes any claims of misconduct very seriously. In response to allegations of data manipulation in JNeurosci 2012;32:9773-9784 the Journal requested raw data, including images of original, uncropped Western blots. The Journal determined that there was one duplicated panel in Figure 8 and a Corrigendum was requested and will be printed. No evidence of data manipulation was found for Western blot data.”“
Hoau-Yan Wang , Kalindi Bakshi , Maya Frankfu rt , Andres Stucky , Marissa Goberdhan , Sanket M Shah , Lindsay H Burns Reducing amyloid-related Alzheimer’s disease pathogenesis by a small molecule targeting filamin A The Journal of neuroscience (2012) doi: 10.1523/jneurosci.0354-12.2012
On 10 November 2021, an Erratum, sharing “ uncropped blots” purporting to be unadulterated raw data. Alas, those pictures were fake, and not even very professionally, as Bik and other sleuths swiftly spotted:

Maybe the Journal of Neuroscience editors and SfN leadership hold Cassava stock? In any case, they don’t reply to my emails and apparently only talk to Cassava: one doesn’t hear from them in this regard otherwise. Important is that SfN obviously can’t wait to sacrifice some Alzheimer’s patients to Cassava’s crookery. Because a phase 3 clinical trial (NCT04994483) with 750 participants is coming up!
Safety first!
Now, the Cassava faithful keep crying, so what if the data is fake? So what Cassava has been constantly lying? The drug was declared as safe, so bring us hundreds of Alzheimer’s patients to experiment upon for the upcoming phase 3 clinical trial!
The CassavaFraud team comments in this regard:
“Is Simufilam really safe? Probably? Maybe?
● Cassava claims Simulfilam is safe, but data suggests a cavalier attitude towards safety, a calculated avoidance of critical studies, and dependence on unreliable investigators
● Simufilam doses administered are millions of times higher than should be required based on the purported mechanism and pharmacokinetics. Simufilam might be safe.. but only because it is inert and does not bind its supposed target.
● Cassava’s Phase 1 study tested only a single administration of the drug
● If the Ph2 biomarker studies were manipulated or fabricated, as the data suggests, how can safety results from the same trials be relied upon?”
Thus, we actually don’t really know if Simufilam is safe, and we also don’t know what happens at different doses. But even if it was safe as a placebo pill: Just because a treatment is not known to cause direct damage it doesn’t mean it can’t hurt. Patients participating in a bullshit trial are not available for more serious clinical studies, and they receive an experimental bullshit drug instead of their usual standard therapeutic regimen which would have surely been a better option. Just because there is no treatment for Alzheimer’s doesn’t mean patients can automatically become guinea pigs for greedy crooks to experiment upon.
And Cassava people are most definitely crooks, cartoonishly so. As the four sleuths of CassavaFraud quipped:
“even Theranos didn’t submit fake data to FDA”
Update 21.12.2021
One journal backs down, another steps into it.
Journal of Neuroscience issued an Expression of Concern for Wang et al 2012, meekly announcing that “JNeurosci will await the outcome of [CUNY] investigation before taking further action.“
But now Neuroscience embarrassed itself to please Cassava, regarding this paper:
H.-Y. Wang, E. Friedman, M.C. Olmstead, L.H. Burns Ultra-low-dose naloxone suppresses opioid tolerance, dependence and associated changes in mu opioid receptor-G protein coupling and Gbetagamma signaling Neuroscience (2005) doi: 10.1016/j.neuroscience.2005.06.003
One example:
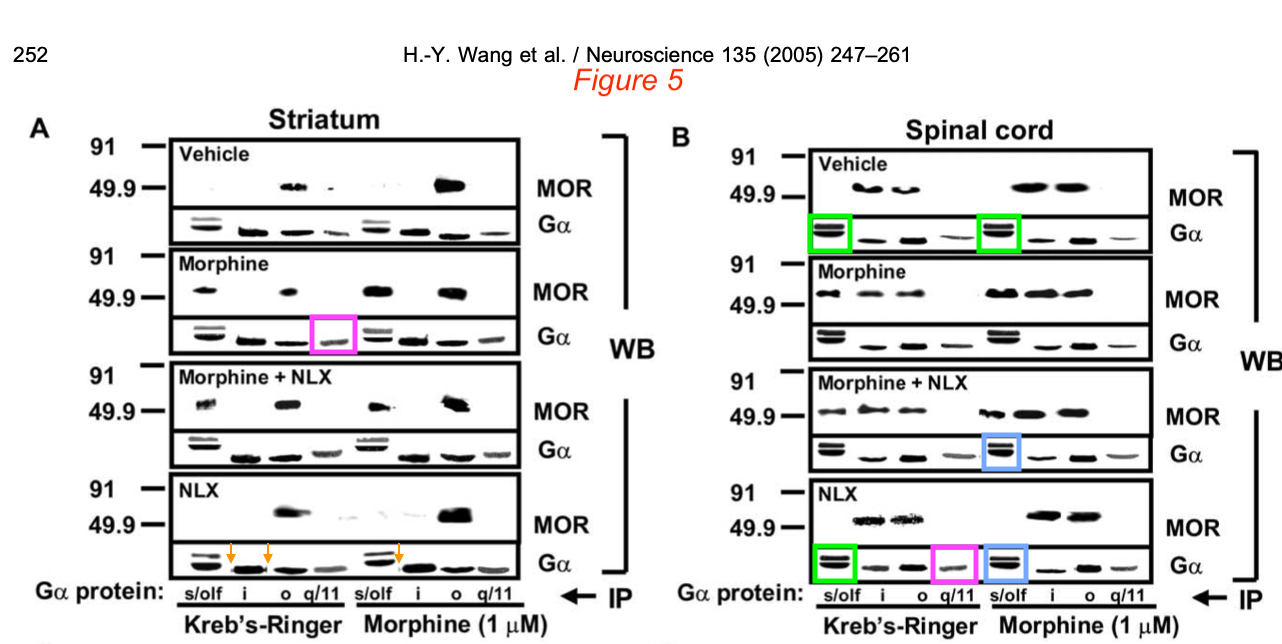
So the Editors-in-Chief Juan Lerma, professor in Spain, and Jerome Sanes, professor at Brown University, USA, issued a note on 20.12.2021:
“In response to allegations of data manipulation in an article published in Neuroscience Vol 135, Issue 1, 2005, Pages 247-261, and following COPE (Committee on Publication Ethics) guidelines, the journal asked the authors for images of the original, uncropped Western blots from this study. After careful examination of these original material, Neuroscience found no evidence of manipulation of the Western blot data or other figures of this publication. […]”
These once again very unconvincing images of “raw data” were shared with the public. Elisabeth Bik swiftly exposed them as utterly fake.

You see, Cassava fraudsters are too dumb to even fake data half-way convincingly. Yet it suffices. There is a big bunch of esteemed neuroscience professors in editorial positions who keep falling for it. Aren’t you worried about the intellectual capacities of our science elites? I am.
PS: Cassava of course issued a press release about the Neuroscience editorial note, titled:
“Science Journal Finds No Evidence to Support Claims of Data Manipulation in 2005 Publication”
They also cite in their support an anonymous blog, ad-science.org. This blog by “A Neutral Party Who Is Expert In The Field“, who declares to have “a Ph.D. degree in Molecular Biology and has been an academic researcher since 2003. His laboratory studies cancer and other human diseases and routinely run western blots (~1,000/year) for their studies“, was set up in September 2021, with all its posts defending Cassava, occasionally attacking Elisabeth Bik. Not very convincing, no?




“They developed a senescence eigengene approach to identify rare, senescent, cells within large, diverse populations of postmortem human brain cells. Eigengenes, the team noted, are useful when no single gene reliably captures a phenotype, like senescence.
By profiling tens of thousands of cells from the postmortem brains of people who had died with AD, the team found that approximately 2% of cells were senescent cells.”
The concept of “senescent neurons” is a curious one. Orr’s press-release defines ‘senescence” thus: “Senescent cells—cells that have stopped dividing—continue to build up in tissues throughout the body.” Of course all neurons stop dividing, so all neurons are senescent, and it is true that if you kill all neurons then the symptoms of AD soon stop.
Orr seems to be creating a new class of cells, “Really senescent neurons”. This class has no a priori definition or biomarkers. Instead, they collected a lot of gene-expression data for a lot of cells and ran it through a kind of Factor Analysis to group the cells in empirical groups (factors, “eigengenes”), where each group is defined by a particular constellation of gene expression. Then they selected the group of cells showing the largest proportion of tau-opathy tangles, and declared “Behold! This group are really senescent! Therefore senolytic drugs will kill just these neurons, and leave the rest of your brain OK!”
This is nonsense on stilts. It is a method that will always provide a positive result.
LikeLike
What is great with their stat is that the manipulation is so stupid it’s overtly evident they are just cheating.
LikeLike
Still not a reason not to put cancer- free people on a chemotherapeutic to see what happens.
LikeLike
For anyone that wants to see how credible and honest this Jesse Brodkin is (since our journalist expert on neurology cited him in his article and probably runs his fan club) – have a look at this twitter thread. Jesse was clearly wrong and couldn’t even admit it, and after I badgered him for a couple days without any responses, he had to run to YMB for a respite. Classic case of someone that can dish it out but can’t take it, just like Leonid. Just like all the SAVA shorts.
LikeLike
Huh? What exactly are you protesting against?
Also, as a non-American, i am a bit confused after learning about IMIC. Is Cassava a kind of a far-right evangelical religion where Remi Barbier is worshipped as the second coming of Jesus? You folks sure behave like this.
LikeLike
“David Sinclair made off with $750k from GlaxoSmithKline for his Sirtis scam, and he is now running another profitable quack business, Elysium Health.”
If GSK only had been that lucky. ~U$750m it was. You’re off by a magnitude. ;p
LikeLike
You are right! Wrote in a hurry!
LikeLike
Sounds like the same lame words of the people you like to bash. Perhaps the rest of your article has the same level of accuracy? I would love to short you if it was possible! “Wrote in a hurry!” Perhaps that should be the title of this piece.
LikeLike
Larry, are you funny in your head? I mistyped a letter, not faked several clinical trials and a series of research papers, honey.
LikeLike
The Sinclair kinda grift according to another grifter?
https://twitter.com/i/broadcasts/1YqJDqMgkWQxV skip to 3m14s
LikeLike
Brenner and Sinclair are direct competitors on the NAD+ scam, pardon, health supplement market.
LikeLike
Someone may want to retract their joint press release with Cassava now:
Expression of Concern: Wang et al., “Reducing Amyloid-Related Alzheimer’s Disease Pathogenesis by a Small Molecule Targeting Filamin A”
Journal of Neuroscience 17 December 2021, JN-ERR-2306-21; DOI: https://doi.org/10.1523/JNEUROSCI.2306-21.2021
“JNeurosci is publishing an Expression of Concern for the article, “Reducing Amyloid-Related Alzheimer’s Disease Pathogenesis by a Small Molecule Targeting Filamin A,” by Hoau-Yan Wang, Kalindi Bakshi, Maya Frankfurt, Andres Stucky, Marissa Goberdhan, Sanket M. Shah, and Lindsay H. Burns, which appeared on pages 9773–9784 of the July 18, 2012 issue. The editors have been made aware of concerns about Western blots in this study, including those published with the article’s erratum (Wang et al., 2021). These and other concerns are currently under investigation by the academic authorities at the City University of New York (CUNY). JNeurosci will await the outcome of that investigation before taking further action.
LikeLike
Cassava Sciences is a public company and so obligated to provide accurate timely information to investors. The company’s silence is deafening.
BTW Cassava Sciences, do you know where your clinical investigator is? Where has Evelyn Lopez-Brignoni, M.D., she of FDA warning letter fame, gone? She vanished from the list of “our dedicated medical professionals” on the International Medical Investigations Center (IMIC) website:https://imicinc.com/about-our-office/
This trial ends in 2023: https://www.clinicaltrials.gov/ct2/show/NCT04388254
Who is the qualified clinical investigator selected to replace Dr. Lopez-Brignoni?
When did Cassava Sciences report the FDA warning letter to Dr. Lopez-Brignoni to the National Institute on Aging?
The 13 subject trial publication raises questions: http://www.aging-news.net/wp-content/uploads/2020/02/Aricle-H.Y.-Wang-1.pdf
The paper says there were 5 trial sites but the study registration indicates only 2 sites, neither of which is IMIC, Dr. Lopez-Brignoni’s site.https://clinicaltrials.gov/ct2/show/NCT03748706
The trial requires an overnight confinement; the 2 sites listed have overnight facilities but so far as can be determined from their website, IMIC does not: https://imicinc.com/for-sponsors/
Where did the subjects and caregivers entrusted to Dr Lopez-Brignoni stay overnight?
The consent form (at the end of the protocol) doesn’t mention sending blood or CSF to Dr Wang at CUNY https://clinicaltrials.gov/ProvidedDocs/06/NCT03748706/Prot_ICF_001.pdf
The paper (p.5) says “All biomarker assessments were performed blind to treatment day; samples were coded prior to testing.” Please correct me if I missed this but the protocol doesn’t appear to describe coding, rather only sending samples from the sites to Dr Wang and Worldwide.
LikeLike
Pingback: Facts and Fiction of Cassava Sciences – For Better Science
I recently heard from CUNY that the FOIA request cannot be filled until January 7th (not Christmas, as I had previously been told)… rest assured I will let the public know what I get ASAP.
LikeLike
What was their reasoning? When did you file your initial request? Who was the CUNY representative who told you this?
LikeLike
When did you file your initial FOIL? With whom? What was the reason given for the delay? What CUNY representative told you the fulfillment of the request would be delayed?
LikeLike
Well, Jesse? We’re waiting. You never responded to my questions. I’d love to see screenshots of these emails about the delays from CUNY posted to Twitter.
LikeLike
Great Column Leonid! I send it to all my friends who need a comprehensive summary or where we are to date. I appreciate your effort to get the word out on this terrible predatory and unethical company. Keep up the great work.
LikeLike
If your friends are into stocks and my reporting saved or earned them money, please tell them I have a “donate” button 😉
LikeLike
Confused person asks: Pain Therapeutics, Inc. Investor presentation dated October 04, 2018, p. 36 identifies Lindsay H Burns as VP, neuroscience since 2001
https://www.sec.gov/Archives/edgar/data/1069530/000106953018000055/ptie-20181004xex99_1.htm
A search of all Cassava Sciences’ SEC filings back to 2001 indicates the 2018 investor presentation is the first mention of Dr. Burns’ position with the company:
https://www.sec.gov/edgar/search/?r=el#/q=Lindsay&dateRange=all&ciks=0001069530&entityName=CASSAVA%2520SCIENCES%2520INC%2520(CIK%25200001069530)
If Dr Burns has been a vice president of the company since 2001, wouldn’t this information be in company filings before 2018?
LikeLike
If fraud is the case then why haven’t more medical journals come out in agreement or the fda? U do say that everyone can see the fraud on the western blots, but the only people talking about this is those with a financial intrest in shorting Sava.
I’m also confused as why both Dr’s that posted the cp didn’t include their financial position on Sava. If I was to find something that implied fraud I definitely wouldn’t leave out important facts about me shorting Sava. I also wouldn’t have put whistle-blower in the cp when I know I’m not getting this info from an employee of Sava.
A lot of things don’t add up for the ones promoting this accusation and when I ask the same thing on Twitter I get blocked by oeople like Jesse. Curious to see if this gets deleted as im only looking for the truth and I would think anyone claiming fraud would provide this info vs blocking someone because they don’t want to answer questions. If it was me I would have said everything in the cp vs 2 days after its release. Just seems like a tactic to ensure the sp tanks, so its about money and not the truth.
LikeLike
Rizzy dear, I don’t know about others, but I don’t hold any stock, of Cassava or anything else. Neither do I live in New York as your friends here claim.
https://www.dropbox.com/s/4horn6avsmn1h82/Petition_to_DOJ_on_behalf_of_SAVA_v8.pdf?dl=0
LikeLike
The first complaint in Cassava’s DOJ Petition is that someone inside the company engaged in insider trading, so it seems premature to rule out the possibility of a whistle-blower.
“The first instance of illegal short activity was in May 2020. As investors awaited the release of the
biomarker data for SAVA’s Alzheimer drug Simufilam, some investors noted a large purchase of 5000
puts at $5 for .05 each and 2500 puts at $7.5 for .10. This is highly unusual options activity, indicative of
insider knowledge. Indeed, a couple days later, Cassava announced failure of biomarker testing and the
stock crashed from $9 to $2 per share.”
LikeLike
What if some people from inside the company deliberately compromised data to short sell the stocks? That would be evil genius (I am not suggesting Cassava has actually any good product, it’s all bullshit). I wonder if they are just so inconpetent some of their scientists are just trolling to make money.
LikeLike
Remi Barbier is going to make us all die of laughter. This transcript is supposed to be an interview with a physician who knows two people in the phase 2 trial (not his patients):
https://ad-science.org/2022/01/09/dr-douglas-baker-shares-his-personal-story-about-cassava-sciences-trial-patients/
The trial has a 12 month open label period followed by a six month randomized withdrawal and ends with a final 6 month open label period.
The more they hype the alleged wonderful improvement in the open label period, the more likely subjects will drop out when/if randomized to placebo at 12 months. Or they won’t notice.
And of course the subject whose family was flying from Ohio to Miami (monthly) to be in the trial has since been moved to the Ohio trial site, right? Because nobody would risk flying with an elderly vulnerable person when COVID rates are so high just so a Miami trial site could continue to collect a fee, RIGHT?
LikeLike
You should supervise your child, before just publishing their theme essay as your own. The crime, is keeping a good drug away from the millions that desperately need it, for the greed of money or attention. Hope you never need something similar.
LikeLike
Hope you never get something simufilar
LikeLike
Over to you Cassava:
What are your plans to ask FDA for accelerated approval for simufilam?
Will you provide an expanded access protocol for patients who are ineligible to enroll in your trials? When can we expect this?
LikeLike
Pingback: Таблетки от старости: как мир ищет лекарство от болезни Альцгеймера
Pingback: Silvain Lesné is a failed scientist – For Better Science
More Alzheimer’s stuff from USF
LikeLike
New updates on the Cassava front – it’s not good and further tanking the stock:
https://www.science.org/content/article/co-developer-cassava-s-potential-alzheimer-s-drug-cited-egregious-misconduct
Click to access cuny_wang_final_report-1697224968927.pdf
Click to access cuny_wang_final_report-1697224968927.pdf
LikeLike
My wild guess why it took 2 years to investigate this crystal clear case of pathetically bad fraud.
CUNY hoped for Cassava stock to go up, and maybe even for FDA issuing approval for simuflimflam.
LikeLike
I meant to include this WSJ reference: https://www.wsj.com/livecoverage/stock-market-today-dow-jones-10-13-2023/card/cassava-stock-sinks-after-university-investigation-into-scientist-fWWMVR4dKZC6cKKjd2fB?siteid=yhoof2
LikeLike
BUY NOW! SIMIFLIMFLAM WORKS! Better than ivermectin!
LikeLike
Perfect timing to short their stock. Making thoss crooks go bankrupt is the only strategy I see, since the authorities won’t move.
LikeLike