For Better Science strives to become the best online resource for Israeli Scientists Jokes, and the pandemic creates new ones almost daily. So here is the follow-up on Schneider Shorts from 4 June 2021, about a very silly COVID-19 cure of “mesenchymal stem cells”, by an Israeli company with a silly name Bonus BioGroup, run by Shai Meretzki and other silly men.
Because it’s almost always men who make the best Israeli Scientists Jokes. Apparently, a combination of male Israeli bullshittery (or in this case, chutzpah) and patriotically-inclined Israeli journalism aiming to entertain American Jewish audience is the right comic relief the world needs during the pandemic.
This is why the biggest clown of them all, Nadir Arber, managed to get all Israeli media, plus Israeli government, and even the then prime minister Bibi Netanyahu push his EXO-CD24 nasal spray quackery miracle cure, and fix a deal with the Greek Prime Minister Kyriakos Mitsotakis to finance and run a clinical trial with Arber’s EXO-CD24 in Greece. Officially because there are not enough COVID-19 patients in Israel, if you believe that. The trial NCT04902183 was unblinded open-label without a control arm, and was celebrated in The Jerusalem Post as a miracle breakthrough after the trial mysteriously lost 2 out of 90 patients. To top it off, it was “overseen by Dr. Sotiris Tsiodras, the national coronavirus commissioner for Greece“, so you see the Israeli Scientists Joke somehow extends to Greek jokers as well.
While Arber is pushing his CD24 cell culture supernatant against everything, and now also against COVID-19, other Israeli Scientists repurpose their own magic medicines. Yes, there are other nasal sprays on the Israeli market of pandemic cures, here a company named SaNOtize, with a Nobel Prize winner on board:
Arber’s colleague at the Tel Aviv University, Shai Efrati, has a high-tech solution to COVID-19: hyperbaric oxygen therapy. His customers leave the pressure chambers not only free of the coronavirus, but also rejuvenated, their telomeres extended into infinity.
And of course, there are also stem cells. In early 2020, I wrote about some other Israeli businessmen of Pluristem and their COVID-19 cure with stem cells, from placenta. They even claimed to have saved dying patients!
Nobody speaks of that miracle cure anymore, COVID-19 clinical trials get cancelled, the Pluristem stock is steadily coasting downwards, so now we have another set of Israeli scientists, with yet another set of dying COVID-19 patients who survived thanks to yet another patented stem cell technology. So here they are, the genius men of Bonus BioGroup:

Like the EXO-CD24 clown Arber, the Bonus BioGroup entrepreneurs are fighting on the so-called “cytokine storm” front. Their “stem cells” are aimed to be applied intravenously, vanquish the enemy “through the secretion of immunosuppressive factors“, and cure COVID-19. Not just this, these “mesenchymal stem cells” from adipose tissue even regenerate damaged lungs:
“By producing keratinocyte, endothelial, and hepatocyte growth factors, MSCs promote the regeneration of lung epithelial cells, prevent the apoptosis of endothelial cells, and contribute to the repair of the alveolar-epithelial barrier damaged by the cytokine storm.”
Bonus BioGroup and their COVID-19 gamechanger features prominently in Israeli media, in Hebrew, English and in Russian. The same trash journalist who previously celebrating COVID-19 crashers of Pluristem wrote in the same trash newspaper The Jerusalem Post in May 2021:
“An Israeli biotechnology company has claimed a 100% success rate in the first 10 patients treated with its drug as part of an early-stage clinical trial at Rambam Health Care Campus in Haifa.The company, Bonus BioGroup, presented the preliminary findings of its Phase I/II trial to peers at the International Society for Cell & Gene Therapy conference in New Orleans last week and shared the results in a statement released to the Tel Aviv Stock Exchange. [….]
Bonus’s MesenCure, which consists of activated Mesenchymal Stromal Cells (MSCs) that are isolated from the adipose tissue of healthy donors, was found to reduce inflammation and alleviate respiratory and other symptoms in patients suffering from life-threatening respiratory distress brought on by COVID-19.”
It was 11 patients actually, not 10, and we learn that one of them died. But because Dr Meretzki decided that “The patient who died did not pass away from COVID-19 but a severe preexisting condition“, that patient didn’t enter the statistics as not to spoil the “100% success rate“.
The clinical trial NCT04716998 in Haifa aims to recruit 35 patients. But there is no control arm. None at all. Since they only recruit patients who can sign an informed consent, intubated (and thus sedated) COVID-19 patients are presumably excluded by default.
There is no peer reviewed paper, but the authors promise one in the future. There is no preprint, and given that most serious journals demand preprinting of COVID-19 material these days, it’s unlike Meretzki et al submitted anything as of yet. In fact, the company Bonus BioGroup doesn’t even have a “publications” section on its website, and they never replied when I wrote to them asking for the list of their publications. No reply.
But I also contacted the company’s founder and CEO Shai Meretzki on LinkedIn, he offered to talk over the phone, but refused to discuss his company’s publications in writing. Maybe that’s because there are very few published results, I found merely 4 Meretzki-coauthored papers on PubMed, none of them COVID-19 related, even distantly. It also seems Meretzki previously earned his PhD at Technion University and Weizmann Institute without having published even one peer reviewed paper, he also refused to explain that when asked.
There is one conference abstract from Meretzki’s company on COVID-19 though. It transpires that Mesencure are not really stem cells, but just some kind of fibroblasts from adipose tissue, or “adipose stromal cells“. Those healthy donors may be some random liposuction patients for all we know. But who cares, the news even made Chinese state media back then.
Now, The Jerusalem Post is at it again, because Meretzki and his Bonus BioGroup treated “six additional patients” with MesenCure, also here one apparently didn’t make it:
“The Health Ministry has approved the expanded use of an innovative COVID-19 treatment that helped 15 out of 17 severe patients who took it to be released from the hospital one day after receiving their final dose.
The drug, MesenCure, has been tested by Rambam Medical Center as part of a Phase I/II trial. The ministry has approved allowing any interested Israeli hospital to take part in the Phase II trial and to use the drug for additional approved patients.
The goal of the expanded trial, which will include a minimum of 50 patients, is to confirm the safety and efficacy of the drug, which was developed by Bonus BioGroup.”
The trial NCT04716998 is still registered to recruit 35 patients though, not 50. Does this all mean the investigators plan to recruit at least 50 COVID-19 sufferers to the uncontrolled open label trial, and issue press releases via The Jerusalem Post while cherry-picking 35 trial participants who qualify by not dying or remaining at ICU?
But worry not. The Jerusalem Post has science to convince you:
“The company’s CEO Shai Meretzki had shared a laboratory image of a healthy lung, a sick lung and lung treated with MesenCure with The Jerusalem Post in May.
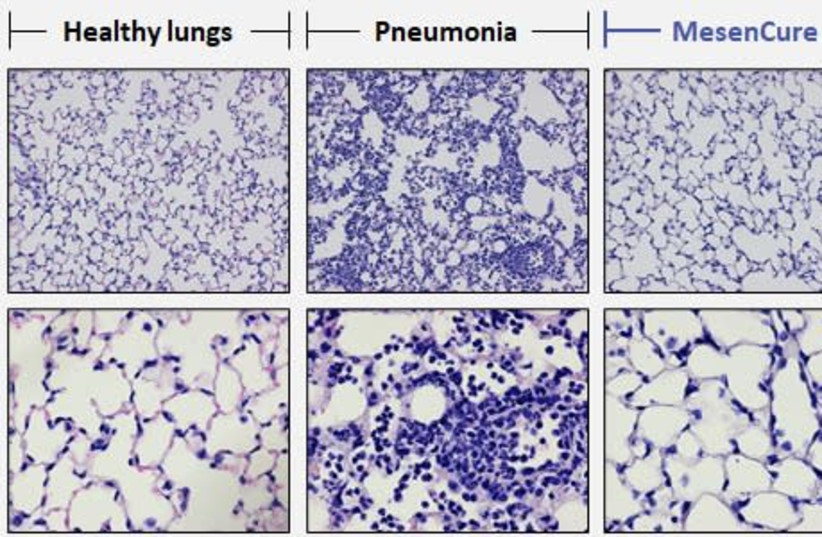
““The treated lung looks almost identical to the normal, healthy lung – complete healing, complete prevention of damage to the lung,” Meretzki said.”
You may think those are the lung biopsies of COVID-19 patients, but of course they are not. As that retched conference abstract admits, those are some mouse models of acute lung injury (not COVID-19 then), specifically mice injected into their chests with LPS (toxic lipopolysaccharides). More importantly, these results remain unpublished.
Before the pandemic, Bonus BioGroup was peddling those same adipose tissue “stem cells” as a therapy for bone regeneration, here may be the clue to the company’s silly name. You know, Bonus, as in “bone”, what a clever pun. The product is patented as BonoFillTM, so to me it seems that a new name had to be invented to repurpose it for the COVID-19 pneumonia, hence MesenCureTM which you are expected to believe is something different from the bone regmed thingy. I suspected that the only difference is that Mesencure is off-the-shelf (or rather out-of-the-liposuction-bag) adipose cells, while BonoFill is autologously derived, meaning extracted from patient’s own fat tissue. But Meretzki protested:
“You got it wrong, MesenCure is completely different product from BonoFill.
Those products use different cells, different source, different activation method, different administration, different mode of action and different clinical indication.
You can get more information about those two products in our website“
I looked at their website, not helpful.
The Jerusalem Post (I love this newspaper, it’s so trash!) even claimed Bonus BioGroup can grow entire bones in a bioreactor. Here is a picture of Meretzki with what looks like his dinner leftovers in a Falcon tube, and look how JP titled it:
(photo credit: BONUS BIOGROUP)”
We learn:
“Founded by Dr. Shai Meretzki and the late Prof. Avinoam Kaduri in 2008, the Tel Aviv Stock Exchange-listed company is behind the world’s first viable human bone graft manufacturing facility, where bone grafts are constructed ex vivo – outside of the human body – from the patient’s own live tissue.
BonoFill, the company’s unique method for growing a three-dimensional and high-density bone graft, requires the isolation of a unique kind of cell from the tissue with the ability to repopulate.
From that cell, a new bone is grown in a laboratory bioreactor mimicking the body’s physiological micro-environment. Within two weeks, a live and viable bone has grown and can be implanted back into the patient.”
What else can Meretzki grow in his stem cell bioreactor, other than whole bones? Tracheas, perchance? Maybe the journalists were drunk when Meretzki was showing them around. Actually, I think both parties were drunk, or whose idea was it to put what looks like a chicken bone into a Falcon tube? Anyway, the JP article is from 2019, and look how successful BonoFill has been:
“Bonus BioGroup’s technology, which boasts a long list of patents including exclusivity for the manufacturing and commercialization of live bone grafts in the United States, is currently undergoing two Phase II clinical trials in Israel for maxillofacial and orthopedic applications.
With positive results, the company expects to start a Phase III US Food and Drug Administration (FDA) trial at the beginning of 2020, with an outcome expected within two-and-a-half years. The company is also aiming to move its listing to the Nasdaq.
“All the patients we have treated within the orthopedic indications have suffered at least three bone graft failures in the past,” Meretzki said. “We take the hardest cases where people have already given up on them. They are all back on their feet or have full use of their hands. One patient, 13 months after he had a bone implant in his leg, completed the Ironman Triathlon.”“
Wow, who needs research papers if you have anecdotes about Ironman Triathlon. In this regard, there is another 2019 Israeli article about the miraculously cured patient who ran an Ironman, complete with picture of BonusBio lab-grown whole bones, this time even bigger ones:

Surely BonoFill clinical trials have been a resounding success so far, right, I mean it has stem cells, bioreactors and all?
Well, the uncontrolled open label phase 1/2 clinical trial NCT03024008 for “Enhancement of Bone Regeneration and Healing in the Extremities by the Use of Autologous BonoFill-II” is still recruiting its 20 patients ever since 2017. The uncontrolled open label phase 1/2 trial NCT02842619 for “Filling Bone Defects/Voids With Autologous BonoFill-II for Maxillofacial Bone Regeneration” is trying the same since 2016 already (both trial registrations were last updated in July 2021). But the uncontrolled open label phase 1/2 trial NCT02153268 for “Filling Bone Defects/Voids With Autologous BonoFill For Maxillofacial Bone Regeneration” did manage to recruit its 11 patients and complete in 2017.
The results of that BonoFill-I trial were published just recently (Tzur et al 2021), one of the exactly FOUR papers with Meretzki’s name to be found. Bonofill-I was declared a resounding success there. Yet the promised phase 3 trial has not been registered as of today. The fact that the two eternally recruiting trials both switched to BonoFill-II instead of BonoFill-I (whatever the difference may be) and yet they still don’t progress anywhere, is also not reassuring.
I am telling you all this so you don’t suspect BonusBio to be bullshitting its investors with bullshit claims, while running bullshit clinical trials which never end while mostly communicating results via The Jerusalem Post. Nothing can be further from the truth! MesenCure/BonoFill must work, it’s a COVID-19 bone crusher with almost 90% success rate! I mean, it’s all over Israeli news! Listen to The Jerusalem Post:
“The company said that the drug could also be used to treat other, similar indications, including, lower respiratory tract infections, asthma and chronic obstructive pulmonary disease, which together, represent a global market expected to exceed $43 billion per year by 2026.”
I’m just not sure that market can be conquered merely with pictures of Dr Meretzki next to leftover animal bones inside lab crockery, regardless of how photogenic they all look.

Make a one-time donation
Make a monthly donation
Choose an amount
Or enter a custom amount
Your contribution is appreciated.
Your contribution is appreciated.
DonateDonate monthly

“Apparently, a combination of male Israeli bullshittery (or in this case, chutzpah) and patriotically-inclined Israeli journalism aiming to entertain American Jewish audience is the right comic relief the world needs during the pandemic.”
Case of female bullshittery.
Jerusalem Post wrote supporting piece.https://www.jpost.com/business/business-features/tamar-tennenbaum-more-than-skin-deep-57069
Was the title a plea for help?
The data:-
Retraction as coauthor.https://pubpeer.com/publications/605A47A08F6E11F60F660CB9F38217
https://pubpeer.com/publications/A77C400952E98CD333A0C372BD5ADD
Figure 8D.
https://pubpeer.com/publications/E7034F1BDF291D662FAFAFE96B671F
Figure 10B.
https://pubpeer.com/publications/5F55A821B7B4499AC4F72B07EB929E
https://pubpeer.com/publications/CE31F5E37AD0F93FECFE62BF43CE4E
LikeLike
A bullshittery pandemic, indeed.
LikeLike
Unfortunately the JPost article has been picked up by the no-masks-no-vaccines-for-us crowd.
Waiting for this to be a new arm in the UK PRINCIPLE trial any day now.
Still not heard a peep about the colchicine arm.
https://www.principletrial.org/news/principle-covid-19-treatments-trial-widens-to-under-50s-adds-colchicine
LikeLike
That is the other quack cure, by Nadir Arber!
LikeLike