Most scientists, like you probably, waste time, space and resources pipetting useless boring crap, without ever founding a pharma start-up and raising some millions for a breakthrough therapy. But, as this 2016 story by the Washington State University titled:
Leen Kawas is on a mission…
…to cure the disease that took her grandmother’s life.
“A scientific discovery that could lead to treatments for Alzheimer’s and cancer drives biochemist and executive Leen Kawas. For her, it’s a personal and professional quest to develop that discovery into innovative, affordable drugs for the millions of people facing those diseases—a quest that started at seven years old, when her grandmother got cancer.
At 30, Kawas ’11 PhD is one of the youngest biotech CEOs in Seattle and, as a woman from Jordan, one of the most diverse. In her first year at the helm of M3 Biotechnology, her small but rapidly growing company, Kawas has been featured on a number of panels as an expert in topics ranging from the technicalities of drug development to how women can succeed as entrepreneurs.
For Kawas, these distinctions are just bonus side effects to the real inspiration that keeps her working 16-hour days: The success of her company could reverse the effects of neurodegenerative diseases like Alzheimer’s.
“I have a passion to solve a problem that is facing millions,” says Kawas. “I could have a job that makes more money, has normal hours, but this is where I should be right now.”
M3 Biotechnology was launched at Washington State University in 2011 by researchers Joe Harding and Jay Wright with support from WSU’s Office of Commercialization. The discoveries that led to M3’s lead drug candidate, MM-201, also have the potential to treat cancer. This is what brought Kawas from her Jordanian city of one million to small college town Pullman. Curing cancer has been her mission since she watched the disease take her grandmother’s life.”
Kawas herself is constantly celebrated as a science genius. Here, a Washington State University story about “Designing Medicine’s Holy Grail“:
“I always have the patient in mind in the drug development process,” Kawas says—which is why developing Dihexa in a cost-effective way is a major priority for both Kawas and Harding and the third member of M3’s executive team, Jay Wright. That is also one of the reasons M3 Biotechnology has proven so attractive to investors.“
The M3 company is now called Athira Pharma, Dr Kawas is the company’s CEO. The luckiest of Washington State University’s students are invited to apply to do an internship in her company. For some reasons, the two M3/Athira founders, the Washington professors Joe Harding and John Wright, are nowhere to be found on the official board of directors, not even on the advisory board. In fact, officially it is Kawas who founded M3/Athira now:
Athira presently runs two phase 2 clinical trials with its Alzheimer’s drug ATH-1017 also registered as NDX-1017. We learn that
“No preclinical studies of NDX-1017 have been published. The drug may be related to N-hexanoic-Tyr-Ile-(6) aminohexanoic amide, aka Dihexa, U.S. Patent 8598118B2. This small-molecule, brain-penetrant angiotensin IV analog was invented by researchers at Washington State University, Pullman. Dihexa activates HGF/MET signaling”
The Phase 1 safety trial with that ATH-1017 aka dihexa drug was done in 2017, funded by Alzheimer’s Drug Discovery Foundation. Yet the results were apparently only summed up at a 2019 conference. In autumn 2020, two phase 2 clinical trials with ATH-1017 began, one with 300 memory loss patients, one with 75.
For that, the company (then still called M3) raised $15.2 million, bringing the total investment to over $26 million. GeekWire reported in 2017:
“The funding includes investments from Seattle biotech veteran Bruce Montgomery and his brother Michael Montgomery, both of whom serve on the company’s board of directors. Other investors include Dolby Family Ventures, the Alzheimer’s Drug Discovery Fund, WFund and Kayne Capital.“
The CEO Dr Kawas was quoted:
“The launch of our first-in-human clinical trials is a significant step toward realizing M3’s mission to develop affordable therapies that modify neuro-degenerative diseases […] Current drugs on the market for Alzheimer’s patients offer only symptomatic relief, whereas we anticipate NDX-1017 will slow, halt and potentially restore lost function. […] The pre-clinical studies suggests we are on the right path, and we are excited to advance a much-needed brain regenerative therapy to alleviate the suffering of millions afflicted by the disease, and their families, around the world“
Now please allow me to teach you how such preclinical drug discovery studies are done properly, so you can learn something.
In a nutshell, it seems many seemingly unrelated diseases can be cured by targeting the Hepatocyte growth factor (HGF) and its tyrosine kinase receptor, Met. This is how ATH-1017 (presently in two Alzheimer’s phase 2 clinical trials) is said to work, it is also being tested in phase 1 for Parkinson’s. The HGF/Met pathway is extremely versatile, if one has the superior intellectual capacity to apply it.
For starters, on cancer:
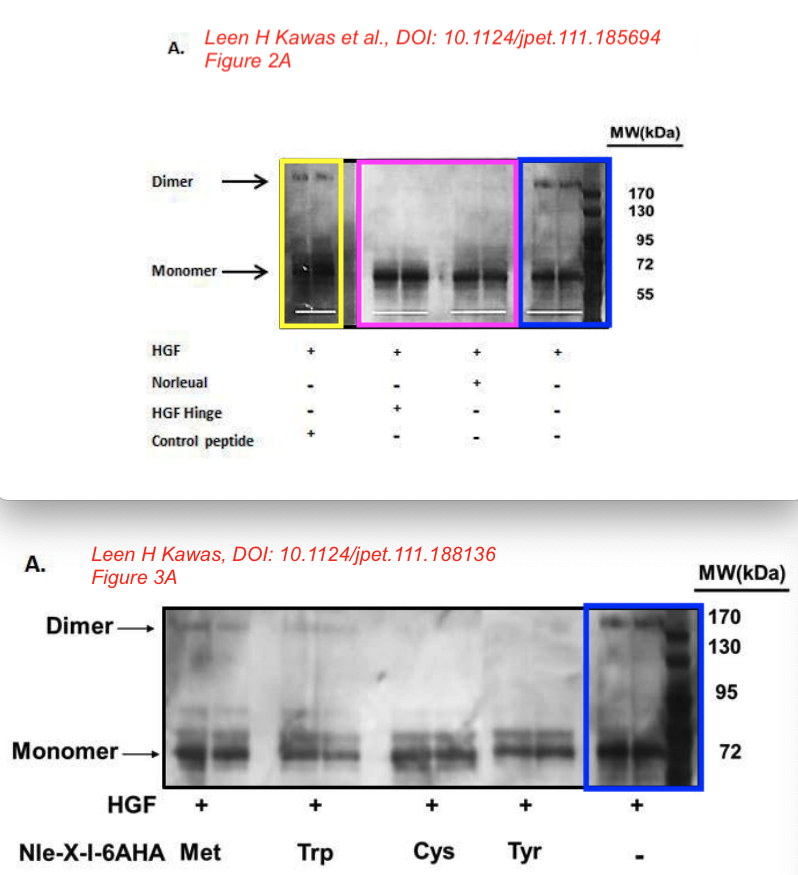
Leen H. Kawas, Alene T. McCoy, Brent J. Yamamoto, John W. Wright, Joseph W. Harding Development of angiotensin IV analogs as hepatocyte growth factor/Met modifiers The Journal of pharmacology and experimental therapeutics (2012) doi: 10.1124/jpet.111.188136
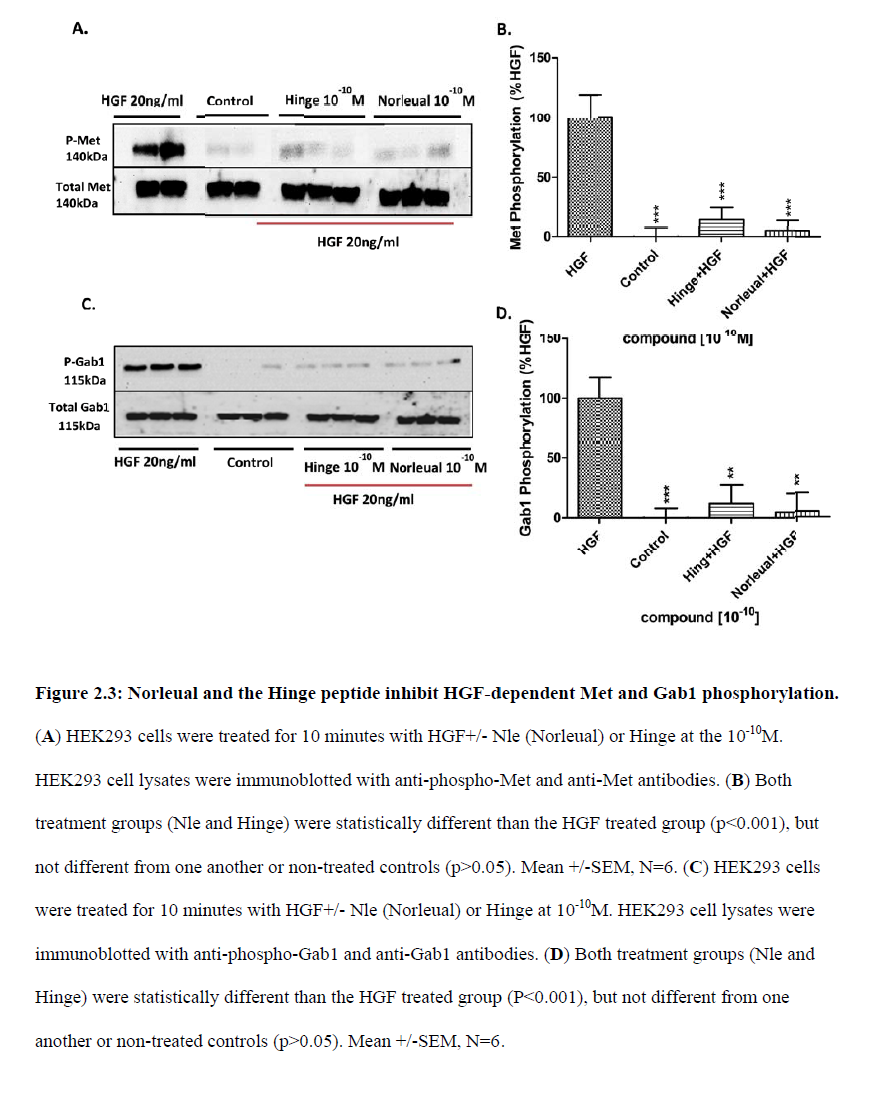
“Together, these data […] offers the potential as therapeutic agents in disorders that are dependent on or possess an overactivation of the HGF/Met system“, which the authors describe as “a common characteristic of many human cancers“. We learn that “D-Nle-X-Ile-NH-(CH2)5-COOH (where X=various amino acids) and norleual [Nle-Tyr-Leu–(CH2-NH2)3-4-His-Pro-Phe] were synthesized using Fmoc-based solid-phase methods in the Harding laboratory“.
Now observe how this cancer drug works:
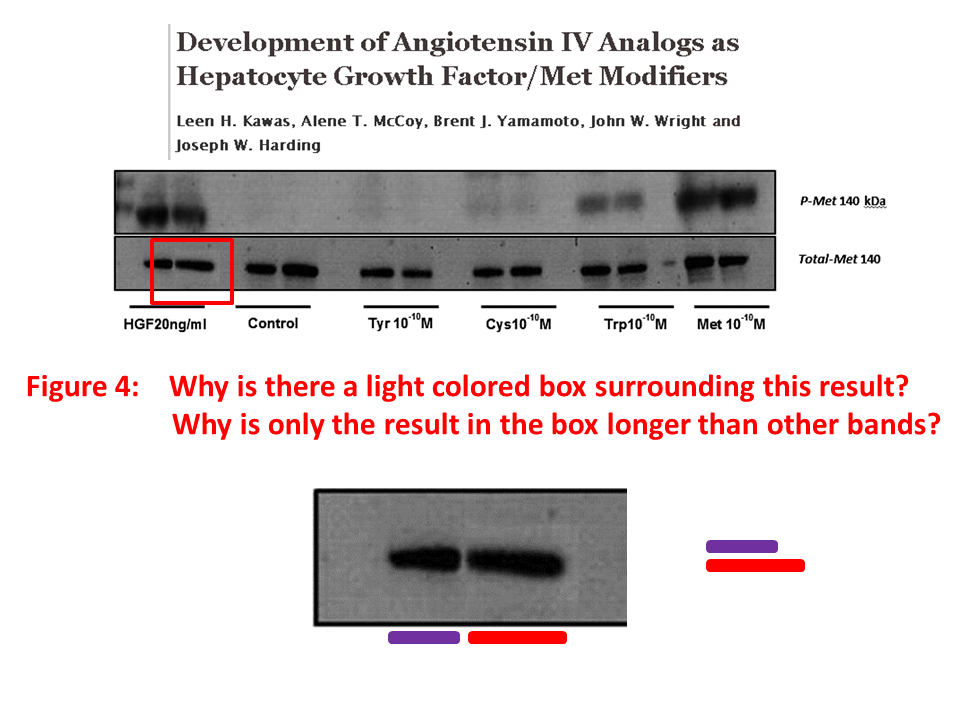
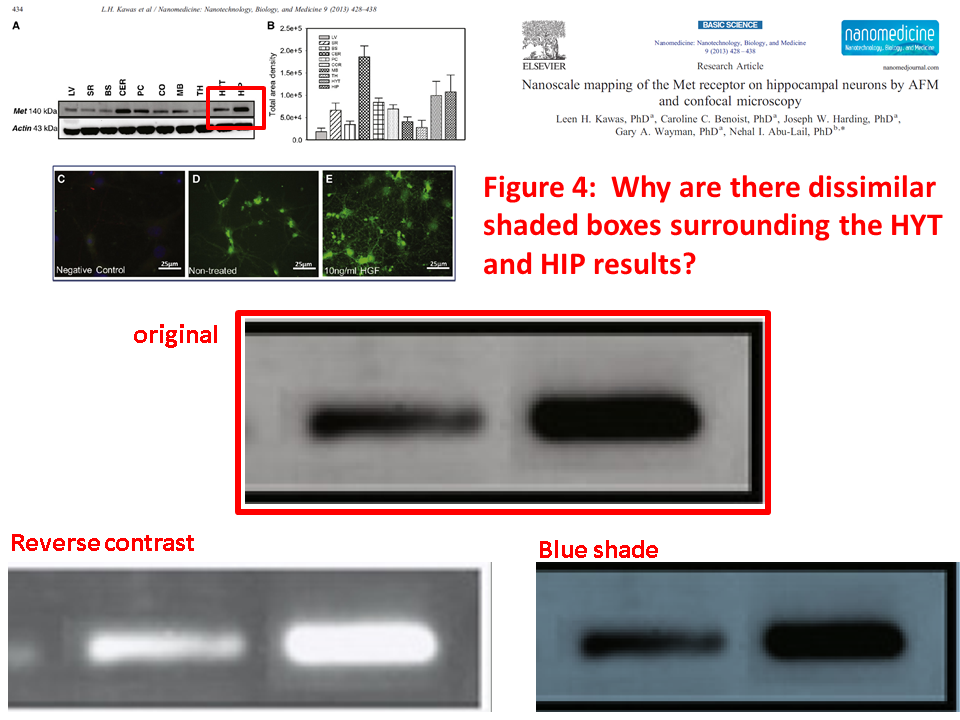
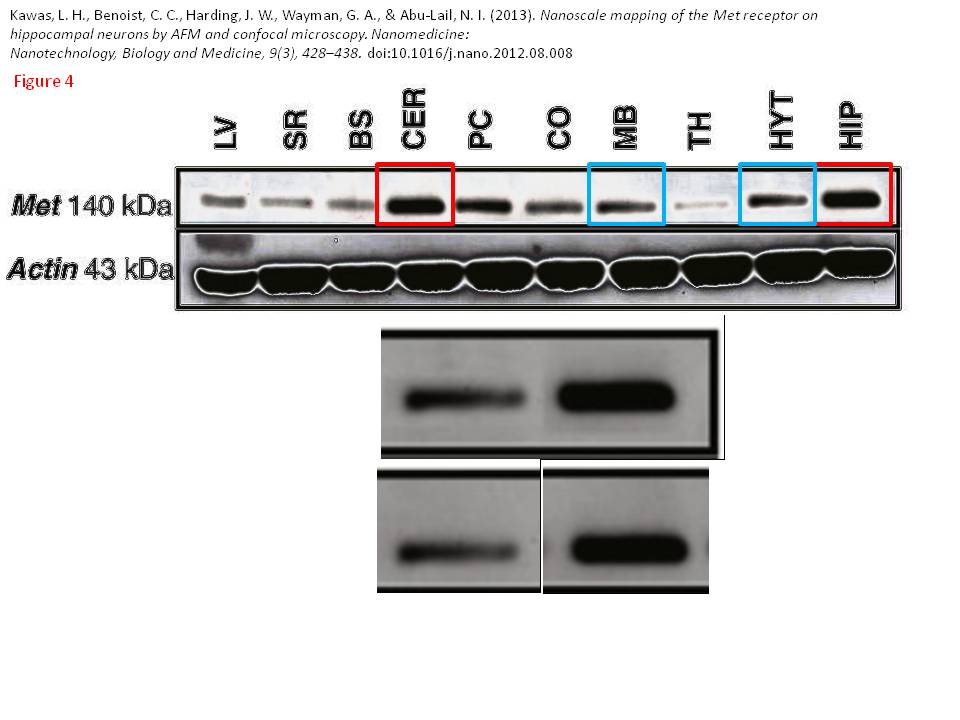
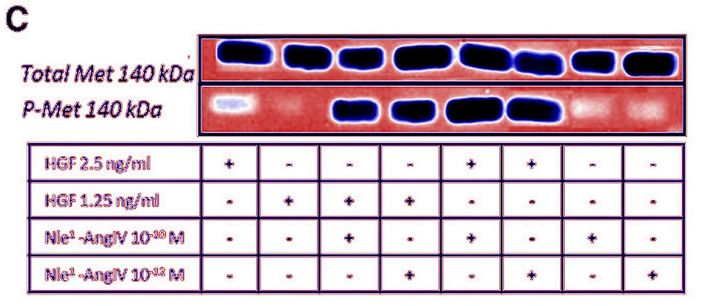
Posted years ago, and a good question: why is there an unnatural box around the bands? “The figure in question appears to be from the PhD dissertation of the first author (page 102)“, below
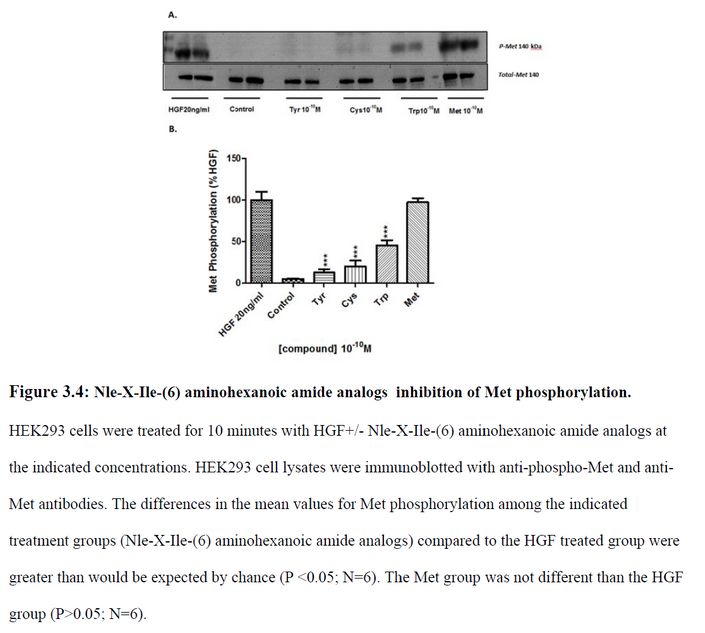
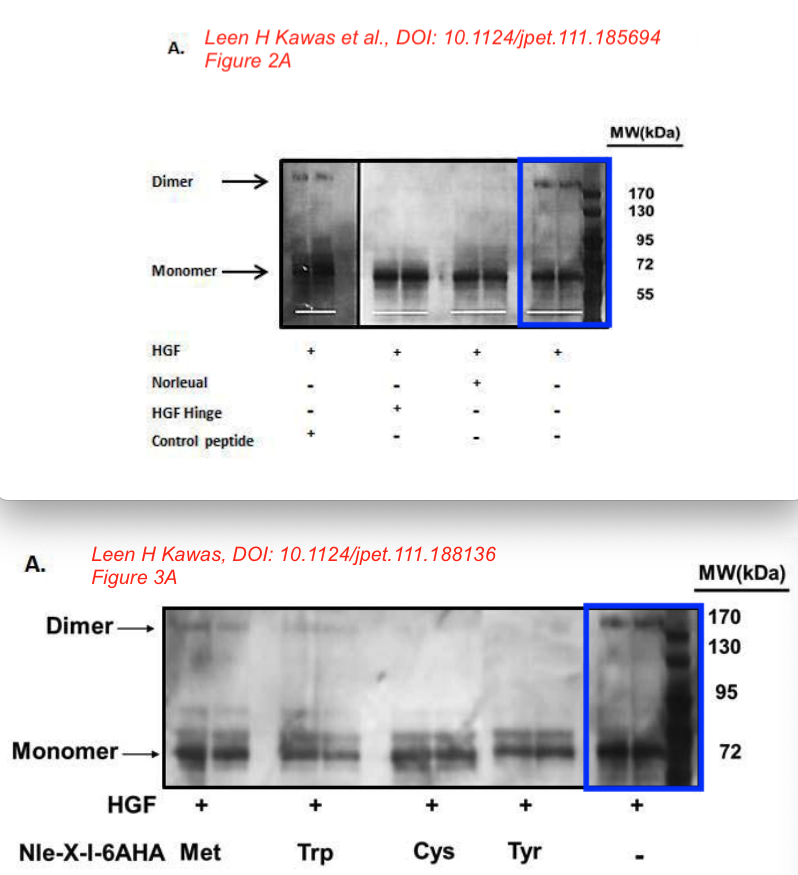
This is the other paper Bik referred to.
Leen H. Kawas, Brent J. Yamamoto, John W. Wright, Joseph W. Harding Mimics of the dimerization domain of hepatocyte growth factor exhibit anti-Met and anticancer activity The Journal of pharmacology and experimental therapeutics (2011) doi: 10.1124/jpet.111.185694
It was also about curing cancer by using the “angiotensin IV analog norleual” on the HGF/Met system, which seems to be at least related to ATH-1017. As the authors inform us:
“The major implication of this study is that molecules targeting the dimerization domain of HGF may represent novel and viable anticancer therapeutic agents; the development of such molecules should be feasible using norleual and the hinge peptide as synthetic templates.“
In 2014, a Correction was issued:
“In Supplemental Fig. 1. a duplicate label for the bottom left image of panel A has been corrected from 10-10 M Hinge to 10-12 M Hinge.”
In Supplemental Fig 2, a duplicate image in the bottom right of panel A has been changed to the correct image.
In Supplemental Fig 3, a duplicate label for the bottom right image of panel A had been corrected from “10-12 M Norleual” to 10-10 M Norleual.”
The authors apologize for any inconvenience.”
Pity that the authors forgot to correct some more figures.
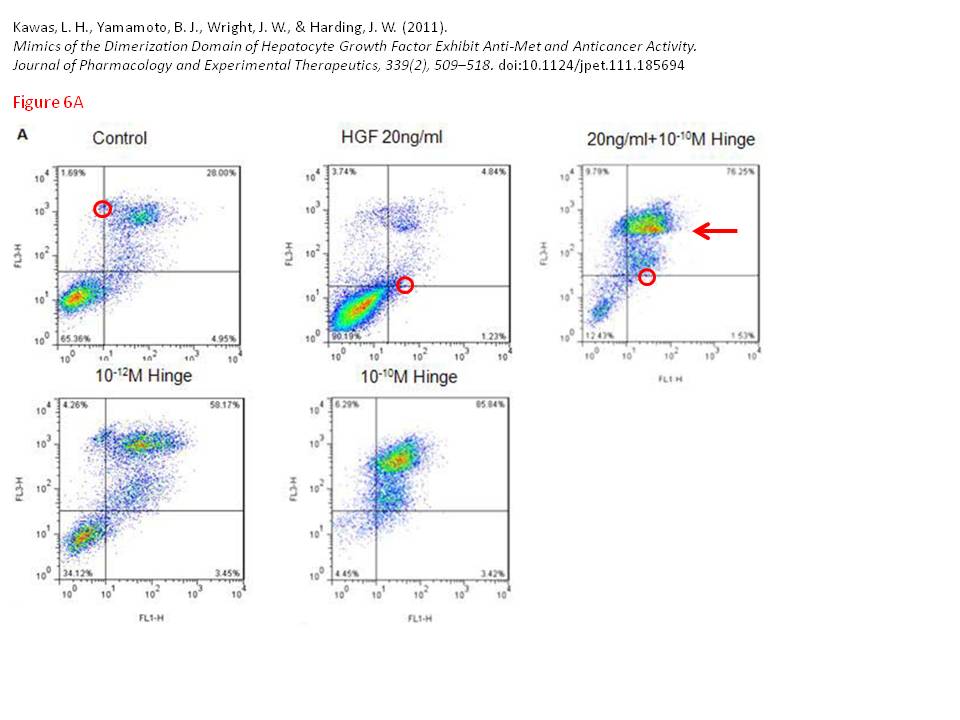
Indigofera Tanganyikensis (typos theirs): “The flow cyotmetry data presented in Figure 6A is also questionable. One of the dot plots show a unusal discontinuity (red arrow). In additon there are data points over the gating line, which is hard to explain since you always put the gating lines after the data is displayed...”
Both above and the following papers declare that Wright and Harding
“…are founders and shareholders in M3 Biotechnology, LLC, which is developing pharmaceuticals based on this technology.“

Image source: WSU
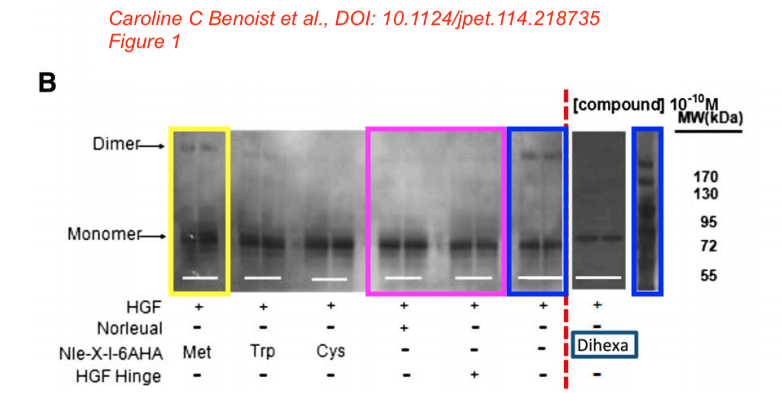
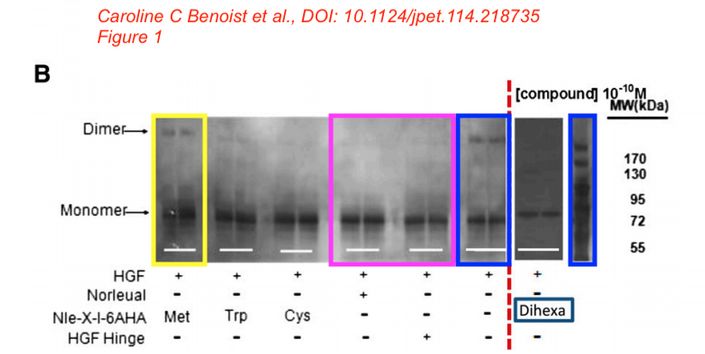
In the next study, Dr Kawas found a cure for dementia, using similar approach as for cancer. Again the HGF/Met system, again with proprietary “AngIV analogs”, this time featuring “procognitive” abilities. In fact, the drug quite distinctly seems to fit the description of ATH-1017, now deployed against Alzheimer’s and Parkinson’s. The scholarly approach here is based on the “we hypothesize” and “we believe” method as the study explains. The peptides were synthesised “in the Harding laboratory“, the funding came from the Michael J. Fox Foundation, a charity founded by the celebrity film actor which finances research into Parkinson’s and other neurodegenerative diseases. Here is this paper on PubPeer, again in same journal:
Caroline C. Benoist, Leen H. Kawas, Mingyan Zhu , Katherine A. Tyson, Lori Stillmaker, Suzanne M. Appleyard, John W. Wright, Gary A. Wayman, Joseph W. Harding The Procognitive and Synaptogenic Effects of Angiotensin IV–Derived Peptides Are Dependent on Activation of the Hepatocyte Growth Factor/c-Met System The Journal of pharmacology and experimental therapeutics (2014) doi: 10.1124/jpet.114.218735
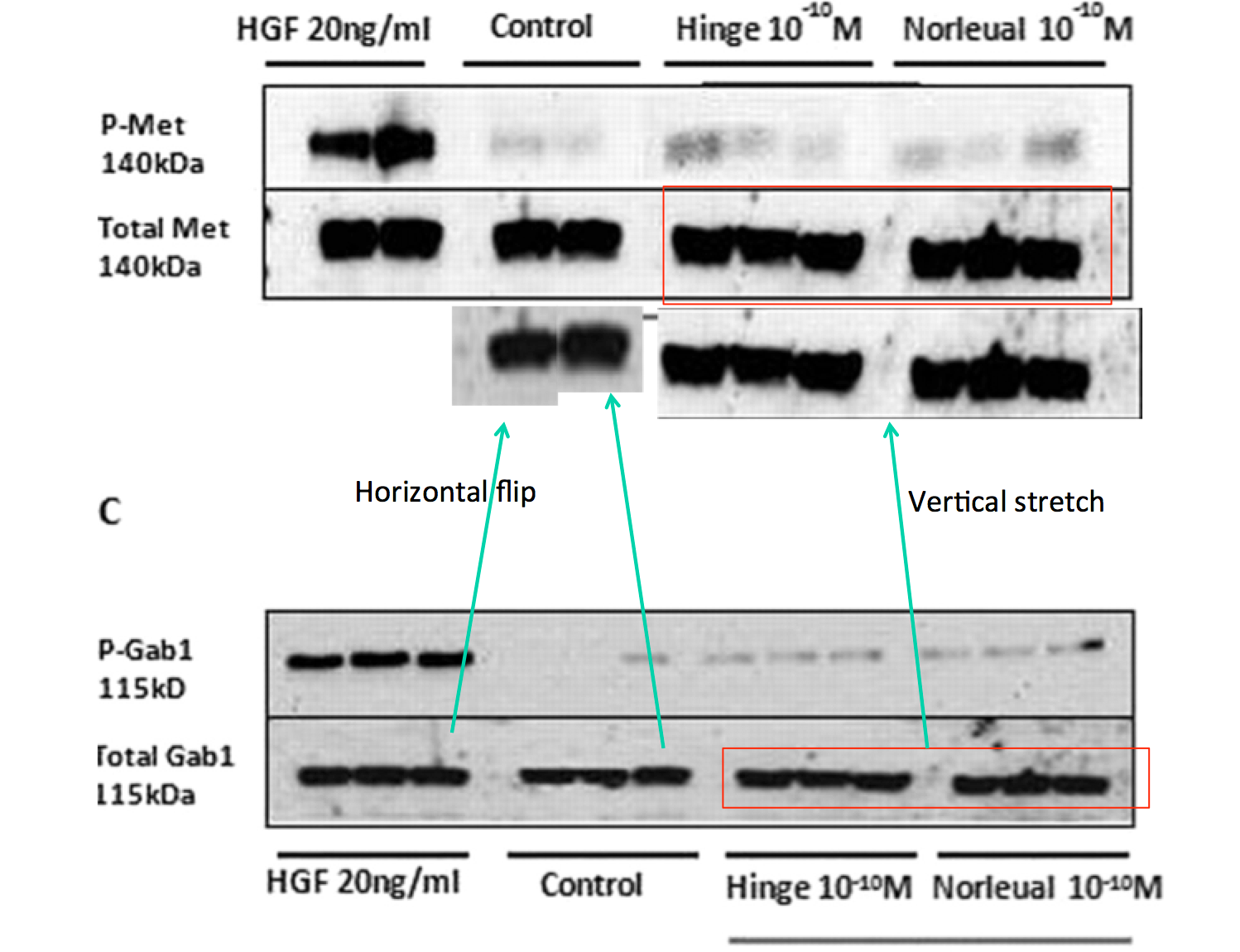
Bik: “Yellow, pink, and blue boxes highlight lanes also visible in two older paper by some of the same authors. The lanes do not always appear to correspond to the same experiments, in particular the four lanes marked with the pink box. Could the authors please comment? Those other two papers are Kawas et al., (2011), DOI: 10.1124/jpet.111.185694, https://pubpeer.com/publications/51C554512CE22267B2E62172DF3DDE , and Kawas et al. (2012), DOI 10.1124/jpet.111.188136, https://pubpeer.com/publications/01199F548BE82EE44C7D395568982B “.
Again, in the same journal and again on curing dementia with proprietary and “procognitive” AngIV analogs. It was funded “by the Edward E. and Lucille I. Lainge Endowment for Alzheimer’s Research, funds provided for medical and biological research by the State of Washington Initiative Measure No. 171 to J.W.W.; the National Institutes of Health [Grant MH086032]; and a Hope for Depression Research Foundation grant to G.A.W.“.
Alene T. McCoy, Caroline C. Benoist, John W. Wright, Leen H. Kawas, Jyote M. Bule-Ghogare, Mingyan Zhu, Suzanne M. Appleyard, Gary A. Wayman, Joseph W. Harding Evaluation of metabolically stabilized angiotensin IV analogs as procognitive/antidementia agents The Journal of pharmacology and experimental therapeutics (2013) doi: 10.1124/jpet.112.199497

Indigofera Tanganyikensis: “In Figure 7A, the PSD-95 staining (green) and the corresponding staining in the merged micrograph are different.“
Yet apparently,
“These data suggest that dihexa may have therapeutic potential as a treatment of disorders, such as Alzheimer’s disease, where augmented synaptic connectivity may be beneficial.“
As reminder, dihexa is ATH-1017, now in two phase 2 clinical trials.
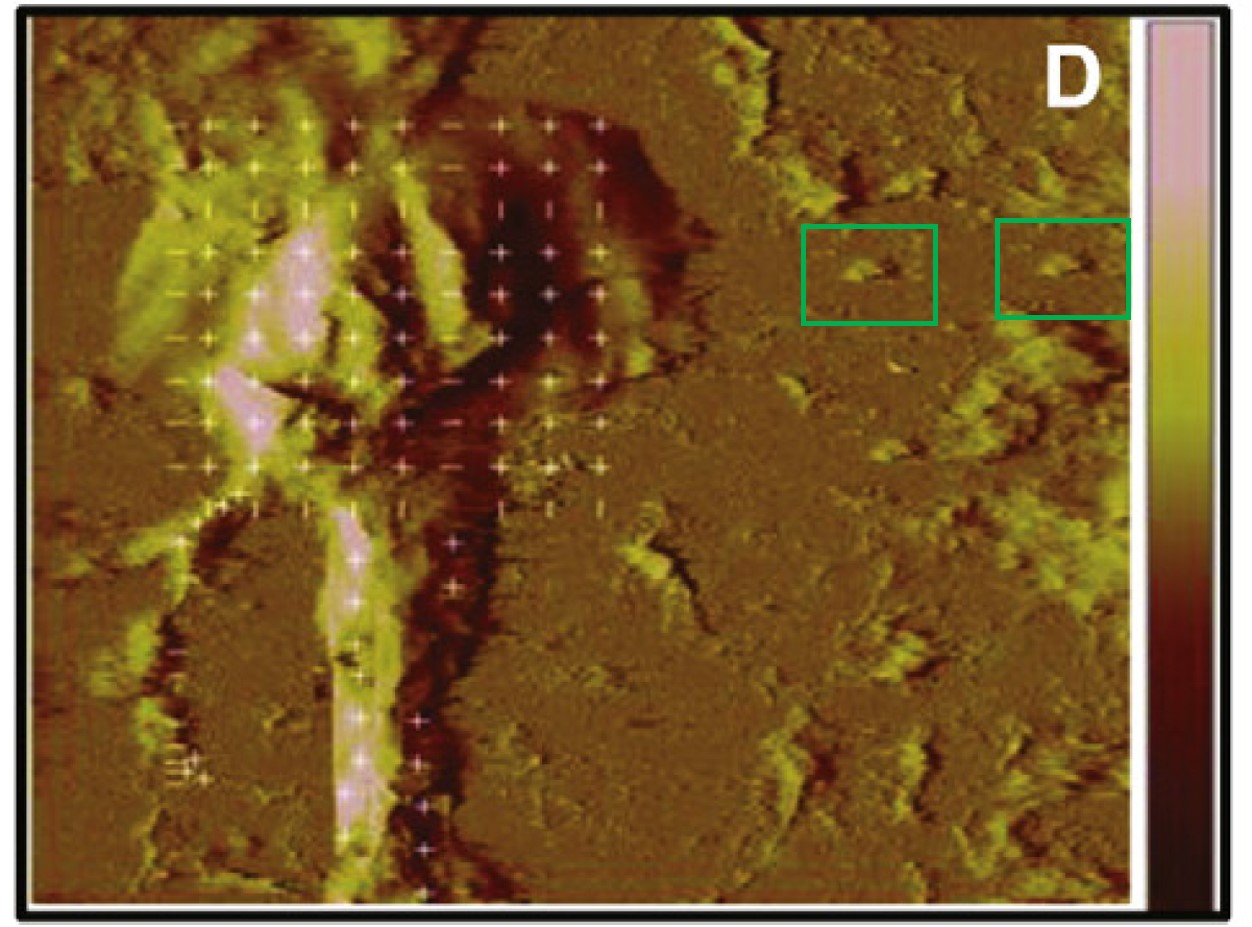
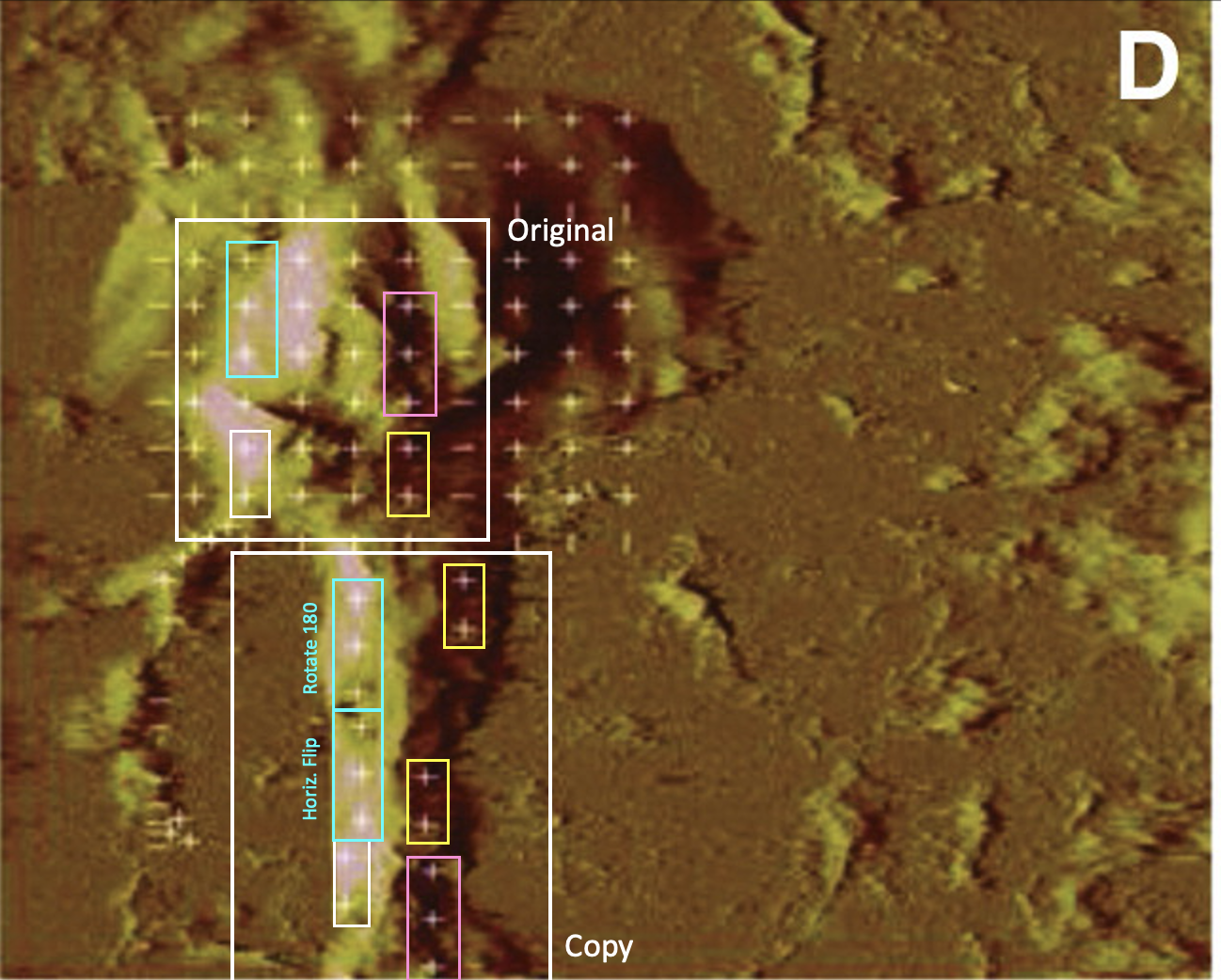
The scholars published their life-saving research in other journals also. here, they “hypothesized that Met should be associated with post-synaptic elements found on dendritic spines” and applied some serious nanotechnology, even atomic force microscopy to study the neuroscience of the HGF/met interaction.
Leen H. Kawas, Caroline C. Benoist, Joseph W. Harding, Gary A. Wayman, Nehal I. Abu-Lail Nanoscale mapping of the Met receptor on hippocampal neurons by AFM and confocal microscopy Nanomedicine : nanotechnology, biology, and medicine (2013) doi: 10.1016/j.nano.2012.08.008
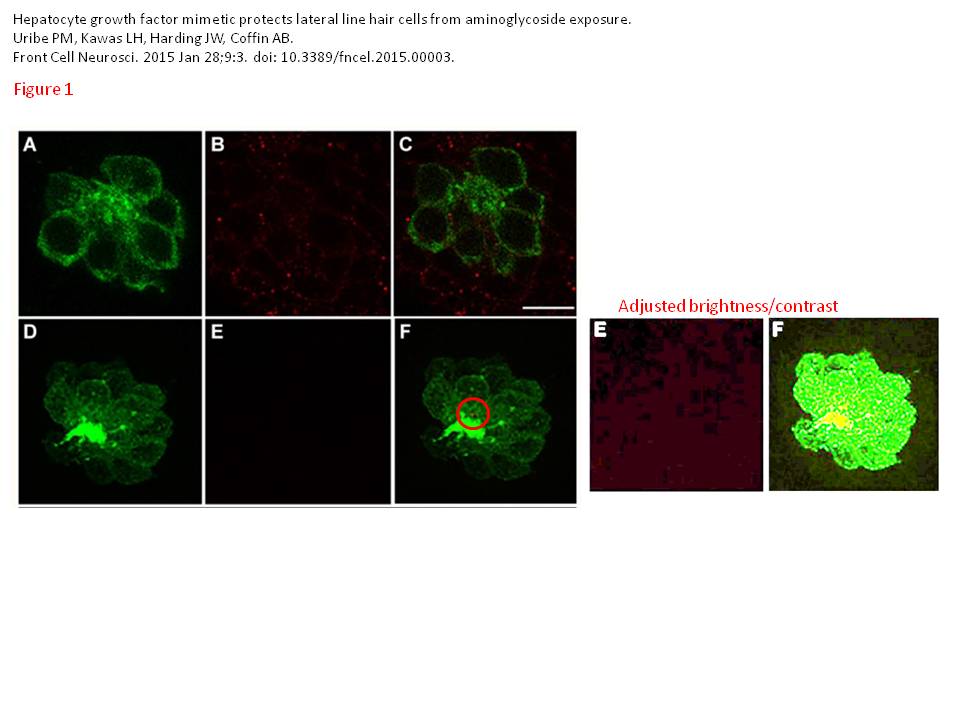
And of course, the scholars published in the best science outlet ever, Frontiers. What did they cure now with ATH-1017, you might ask? Well, hearing loss:
“Due to its conscious design as an orally bioavailable compound, Dihexa is a strong clinical candidate as a hair cell protectant.“
Phillip M. Uribe, Leen H. Kawas, Joseph W. Harding, Allison B. Coffin Hepatocyte growth factor mimetic protects lateral line hair cells from aminoglycoside exposure Frontiers in Cellular Neuroscience (2015) doi: 10.3389/fncel.2015.00003
And what disease did you cure today to get rich, dear reader? You failed scientist.
Dr Kawas and the Washington State University were informed of the PubPeer concerns already a month ago. They were so far to busy to reply.
Update 18.06.2021
A press release from 17 June 2021 by Athira Pharma:
“Athira Pharma, Inc. (NASDAQ: ATHA) (“Athira“), a late clinical-stage biopharmaceutical company focused on developing small molecules to restore neuronal health and stop neurodegeneration, today announced that Mark Litton, PhD, MBA, in his capacity as Chief Operating Officer, has assumed day-to-day leadership responsibilities for the Company, effective immediately.
This follows the Board’s determination to place Leen Kawas, PhD, President and Chief Executive Officer of Athira, on temporary leave pending a review of actions stemming from doctoral research Dr. Kawas conducted while at Washington State University. Dr. Kawas will remain on the Board. The Board has formed an independent special committee to undertake this review. The Company does not intend to comment further on this matter until the review has concluded.
Tachi Yamada, MD, Chair of the Board of Athira, said, “Athira is committed to the integrity of scientific research in its mission to restore neuronal health for those suffering from neurological diseases, so that patients can regain their memories, lives, and family relationships. ATH-1017 was discovered, developed, and patented by Athira on the basis of novel data generated within the Company. The Company is confident in the therapeutic potential of ATH-1017 for treating dementia.“
A new coverage mentions a drop in stock value:
“The temporary removal of Kawas as CEO comes less than a year after the company went public in the Nasdaq in September through a $204 million IPO. Athira posted net losses of $5.2 million in 2019 and about $3.8 million in the six months leading up to its IPO, but losses are typical of a biotech company conducting research and development.
Shares of the company closed at $18.24 Thursday but dropped to a low of $10.26 at 3:05 p.m. PDT in after-hours trading before rising to about $12.27 as of 4:47 p.m. PDT.“
Update 22.10.2021
Leen Kawas has been let go by Athira. The company issued a press release:
“The Company and Dr. Kawas agreed it is in Athira’s best interest to enter this critical next chapter under new leadership. Dr. Kawas’s actions at Washington State University took place many years ago and did not involve ATH-1017, Athira’s lead development candidate,“
Uhm, that last bit is actually untrue. ATH-1017 is the same “Dihexa” Kawas did her PhD on. The press release also informs:
“The Company also announced that its Board of Directors has concluded its independent special committee’s investigation of allegations raised regarding doctoral research by Dr. Kawas conducted while a graduate student at Washington State University (“WSU”).
The special committee’s primary finding was that Dr. Kawas altered images in her 2011 doctoral dissertation and in at least four research papers that she co-authored while a graduate student at WSU, published from 2011 to 2014.”
The phase 2/3 clinical trial with ATH-1017 on 375 Alzheimer’s patients continues.

Get For Better Science delivered to your inbox.
Make a one-time donation
Make a monthly donation
Choose an amount
Or enter a custom amount
Your contribution is appreciated.
Your generous patronage of my journalism will be most appreciated!
DonateDonate monthly



Pingback: La zappa sui piedi del senatore – ocasapiens
Leen “didn’t like being a scientist” and so went into business (5:45). Looks like she’s much better at getting people to invest in her then generating reproducible data.
LikeLike
the Michael J. Fox Foundation, a charity founded by the late celebrity film actor
Ahem.
LikeLike
Oops. Checked Wikipedia, and corrected
LikeLike
I hope the investors are playing with their own money here. Notable lack of due diligence on display.
LikeLike
The fallout:
https://www.marketwatch.com/story/athira-pharma-puts-ceo-on-temporary-leave-shares-fall-26-atha-271623965990
Jesus Christ. She’s a small time artist compared to big time fabricators who have gotten way further up in academia or industry. If she had only been a little more careful…
LikeLiked by 1 person
Meanwhile, at Sanofi:
LikeLike
Geekwire reports:

https://www.geekwire.com/2021/athira-pharma-ceo-placed-leave-shares-plummet/
Completely forgotten to cite any of this:
https://www.geekwire.com/?s=leen+kawas
(“Athira Pharma CEO Leen Kawas accepts the award for Startup CEO of the Year at the 2019 GeekWire Awards. (GeekWire Photo / Kevin Lisota)”)
LikeLike
ATHIRA INVESTIGATION: Block & Leviton Investigates Athira For Potential Securities Law Violations; Investors Who Have Lost Money Are Encouraged to Contact the Firm
https://finance.yahoo.com/news/athira-investigation-block-leviton-investigates-180500927.html
LikeLike
https://www.globenewswire.com/news-release/2021/06/18/2249792/0/en/Athira-Pharma-Inc-Investors-Company-Investigated-by-the-Portnoy-Law-Firm.html
“– The Portnoy Law Firm advises Athira Pharma Inc (“Athira” or the “Company”) (NASDAQ:ATHA) investors that the firm has initiated an investigation into possible securities fraud, and may file a class action on behalf of investors.
[…]
The investigation focuses on whether the Company issued false and/or misleading statements and/or failed to disclose information pertinent to investors concerning the Company’s drug candidate ATH-1017. On June 17, 2021, after the market close, the Company reported that it had placed its CEO, Leen Kawas, on temporary leave. Citing “a review of actions stemming from doctoral research” that Kawas conducted while at Washington State University. Bloomberg News reports that while the scientific hypothesis behind Athira came out of the work Kawas did in graduate school, there is risk that whatever comes out of this investigation could have clear negative implications for how investors view the asset, and/or management credibility.”
https://www.businesswire.com/news/home/20210618005578/en/INVESTIGATION-ALERT-The-Schall-Law-Firm-Announces-it-is-Investigating-Claims-Against-Athira-Pharma-Inc.-and-Encourages-Investors-with-Losses-to-Contact-the-Firm
“-The Schall Law Firm, a national shareholder rights litigation firm, announces that it is investigating claims on behalf of investors of Athira Pharma, Inc. (“Athira” or “the Company”) (NASDAQ: ATHA) for violations of the securities laws
The investigation focuses on whether the Company issued false and/or misleading statements and/or failed to disclose information pertinent to investors. On June 17, 2021, Athira released a statement indicating it had placed President and CEO Leen Kawas on leave and was reviewing “actions stemming from doctoral research Dr. Kawas conducted while at Washington State University.” Based on this news, shares of Athira plummeted by almost 40% on June 18, 2021. “
LikeLike
I am an investor and I am confused regarding the data presented here, was that fabricated intintially ? Does it have anything to do with the product that is undergoing clonical trials ?
LikeLike
Dr Kawas deleted both her Twitter and LinkedIn profiles. Now you know why the tweets above don’t load.
There are more problems with her PhD thesis:
LikeLike
https://news.wsu.edu/2020/12/15/joe-harding-named-2020-national-academy-inventors-fellow/
LikeLike
Good to know Professor Harding still owns Athira.
LikeLike
Your dirt digging is too obvious that it has other motives than just uncover the truth, and tbh, I do not see the correlation between her studies and phase 1 clinical study that was conducted by an independent company called biotrial. Results came out are consistent with what ATH-1017 suppose to do.
The product in these articles is not know to me and I do not know if they were treatment of Dihexa (which is not the same as ATH-1017). The CHF/MET system is well known and its proven by many other scientists not just Dr Kawas.
And yes, no need to post all these law firms because they just go after anyone, they are just leeches
LikeLike
PR Management contact email? What a crappy job you do.
LikeLike
The data from that “independent” study, which was included in the company’s S-1 (see link below, page 129, Figure 20) should be looked at also. It is obvious that the data is HIGHLY SURPRISING to anyone with expertise in those test assays. Maybe Biotrial should be investigated also.
https://investors.athira.com/node/6806/html
LikeLike
These studies are not conducted completely independently, and its clear to anyone with expertise in the assays run by Biotrial (and shown in the Athira S-1, see link, Figure 21, page 129) that the data is HIGHLY SURPRISING. Maybe someone should investigate Biotrial as well.
https://www.sec.gov/Archives/edgar/data/1620463/000156459020041369/atha-s1.htm
LikeLike
That would be this Biotrial?
https://www.sciencemag.org/news/2016/02/french-company-bungled-clinical-trial-led-death-and-illness-report-says
LikeLike
Headline:
Biotech firm Athira Pharma hit by class action lawsuits following image manipulation claims
https://www.geekwire.com/2021/biotech-firm-athira-pharma-hit-class-action-lawsuits-following-image-manipulation-claims/
Both the company and Kawas herself are being sued by inventors.
“All three lawsuits were filed on Friday and allege that the Bothell, Wash.-based company made false and misleading statements to the Securities and Exchange Commission in its filings in preparation for its IPO in September of last year, ultimately misleading investors about “invalid research.”
We learn who originally reported the data manipulations, so reader beware, my above article must have been plagiarized from STAT News.
“On June 17 the news site STAT published a story examining the claims of image manipulation in Kawas’ papers, which first emerged on PubPeer, a service where scientists can comment on the integrity of data in scientific papers.”
But we also learn that both Harding and Wright seem to haveabandoned their company already last year, meaning they won’t be sued. How clever of them.
“Harding resigned from the company’s board of directors in August of 2020. Wright also does not appear to be affiliated with the company per its website and is not associated with the IPO filings.”
LikeLike
https://www.pubpeer.com/publications/B479DFE3C2CB56C05746676B0C95E7#2
Another one to take the drug to patients. Are GLI1 and SHH blots same?
LikeLike
LikeLike
“I regret that mistakes I made as a graduate student many years ago caused any distraction to Athira today,” said Kawas in the letter obtained by GeekWire. “At the time, I was navigating an unfamiliar environment and did not fully comprehend the significance of my decision to enhance the images I used in my research. I want to make clear that the enhancement to images was not a change to or manipulation of the underlying data.”
GeekWire: Internal memo: Ex-Athira CEO says ‘I should have known better’ after data manipulation investigation.
https://www.geekwire.com/2021/internal-memo-ex-athira-ceo-says-known-better-data-manipulation-investigation/
LikeLike
Pingback: Cassava fraud and Alzheimer’s capitalism – For Better Science
Pingback: Silvain Lesné is a failed scientist – For Better Science