A novel Alzheimer’s drug is being tested in clinical trials in USA. The US company Cassava Sciences is ready to earn billions of dollars, convinced to have the game-changer, verified in the lab and in soon in clinic. And then concerns are publicly raised with FDA about falsified preclinical research data with that same drug, funded by that same company, done in the New York lab of their long-term board member, Hoau-Yan Wang. Cassava retaliated with a full–blown attack on its critics via a press release. Which provoked Elisabeth Bik to have a look as well.
On 23 August 2021, the US medical regulatory authority FDA posted this document, a kind of “Citizen’s Petition” by a law firm addressed to the FDA:
Statement of Concern Regarding the Accuracy and Integrity of Clinical and Preclinical Data Supporting the Ongoing Clinical Evaluation of Compound PTI-125, Also Known As Simufilam re Citizen Petition from Labaton Sucharow
This happens when concerned investors employ a law firm to investigate the evidence of research misconduct and get their money back from the accused company. For example, something like this is currently happening to Athira Pharma:
Here, it is about the company Cassava Sciences, founded by its CEO Remi Barbier. The law firm Labaton Sucharow acted apparently on some internal whistleblower information, because there was no PubPeer evidence at that time, and engaged a scientific image integrity expert to verify and summarise the concerns. Part A of the lawyers’ report is an Executive Summary:
“For over 15 years, Cassava Sciences (previously Pain Therapeutics, Inc, PTI) has funded the lab of Dr. Hoau-Yan Wang at City University of New York (CUNY). Together with Dr. Lindsay Burns at Cassava, Dr. Wang has published nearly a dozen papers connecting Filamin A protein with pain and Alzheimer’s disease (AD).
Cassava Sciences created a drug candidate called simufilam (previously PTI-125) that they claim binds Filamin A and has beneficial effects in biochemical and animal models of AD. The studies from Drs. Wang and Burns discussed in this dossier were used by Cassava Sciences to garner NIH grants and to open an investigational new drug (IND) application to study simufilam in AD patients. They form the basic science foundation for two completed clinical trials (phase IIa and IIb) which exposed over 70 patients to simufilam. Cassava Sciences is
currently recruiting 200 additional patients for a follow-up open-label trial.
This report raises concerns about the quality and integrity of the laboratory-based studies surrounding this drug candidate.To preface the analysis that follows, no other labs have confirmed this research connecting Filamin A to pain or AD. No other labs have confirmed that simufilam binds or modifies Filamin A or has effects in AD models.”
In Part B, the lawyers explain that their sources are public (i.e., published research papers) and that
“This letter details a long-standing pattern of seemingly intentional data manipulation and misrepresentation in scientific papers and corporate disclosures authored primarily by Drs. Hoau-Yan Wang, Associate Medical Professor, City University of New York, and Lindsay A Burns, Sr. Vice President of Neuroscience at Cassava Sciences.“
Hoau-Yan Wang is member of Cassava’s advisory board, according to the lawyers for over 15 years already.
What follows soon after, is Part C, where evidence of research fraud is presented. Mostly, it’s about falsified western blots. This was the first criticised paper:
H.-Y. Wang, E. Friedman, M.C. Olmstead, L.H. Burns Ultra-low-dose naloxone suppresses opioid tolerance, dependence and associated changes in mu opioid receptor-G protein coupling and Gbetagamma signaling Neuroscience (2005) doi: 10.1016/j.neuroscience.2005.06.003
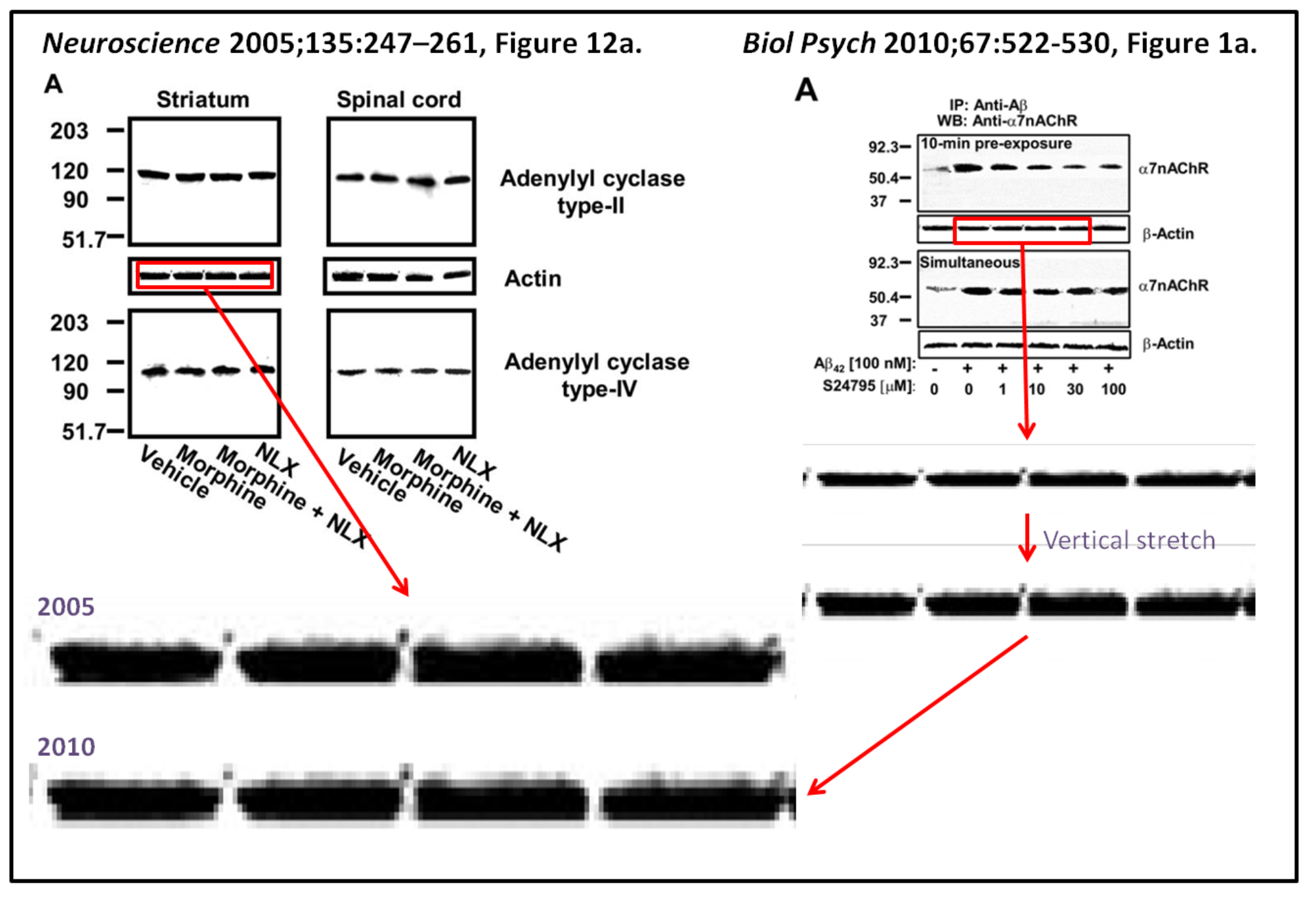
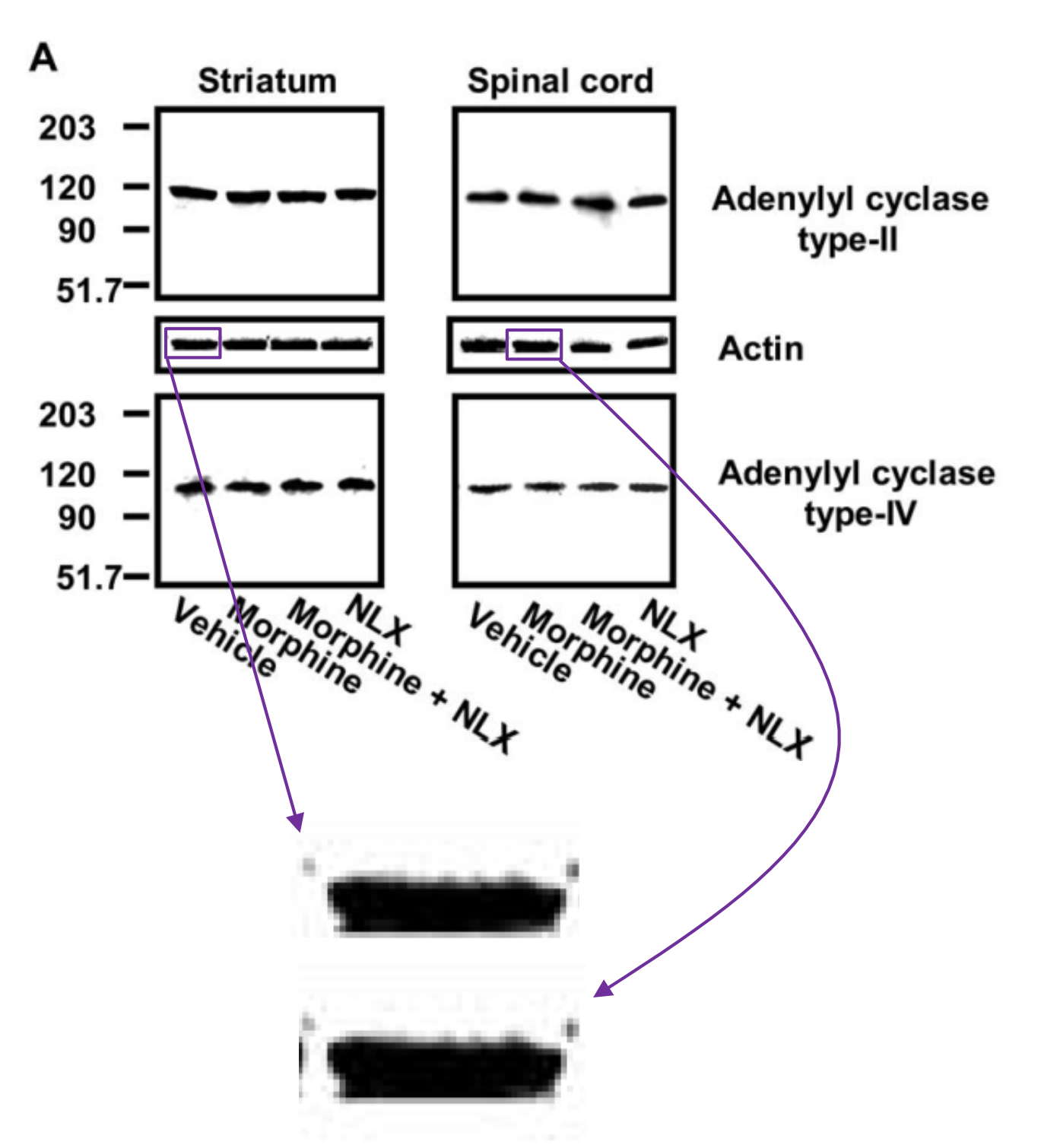
Psychiatry 67:522 contains four bands that closely resemble an image published in Figure 12a
(left) of the Wang and Burns 2005 Neuroscience 135: 247 paper“
Cassava immediately issued a rebuttal to the Labaton Sucharow accusations via a press release, point-by-point retorting to “Fiction” with “Fact”. To me it would kind of make sense actually, if one swaps the Fiction and Fact labels. For example, regarding the western blots like the above, Cassava declared:
“Fiction: Western blots data are identical, more evidence of image manipulation.
Fact: The Western blots bands shown in the allegation are control bands. Control bands are supposed to be highly similar (since they show equal amounts of protein between lanes). Bands show clear differences when expanded. In addition, image manipulation of control bands makes no sense since these would not change the end data.“
Well, that is an old and tired “it’s just controls excuse”. Without controls, a scientific experiment (or a clinical trial) becomes largely meaningless. Imagine Cassava would sell a weight loss supplement instead of an Alzheimer drug. Their advertisement shows a Before and After photos, on the right a lean muscular man, on the left the same picture of same man just photoshopped to make him look fat. What? You aren’t interested in the supplement anymore? It’s just controls! The two men are supposed to be highly similar! Look, the fat guy shows clear differences when expanded. And anyway, manipulation of control male models makes no sense since these would not change the end data, right?

Elisabeth Bik confirmed the concerns reported to the FDA, and challenged by the Cassava statement, searched herself and then found more, much more. She later published a blog post detailing some of the new evidence.

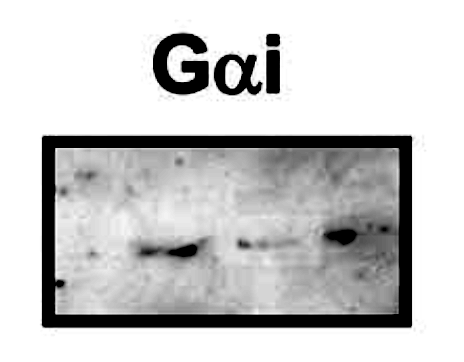
Blue boxes mark very similar panels which differ by brightness only while representing different experiments. On top of that, Bik spotted that the the Gai panel in Figure 2 looks fishy. It does indeed appear that bands were digitally pasted or selectively manipulated otherwise in Photoshop.
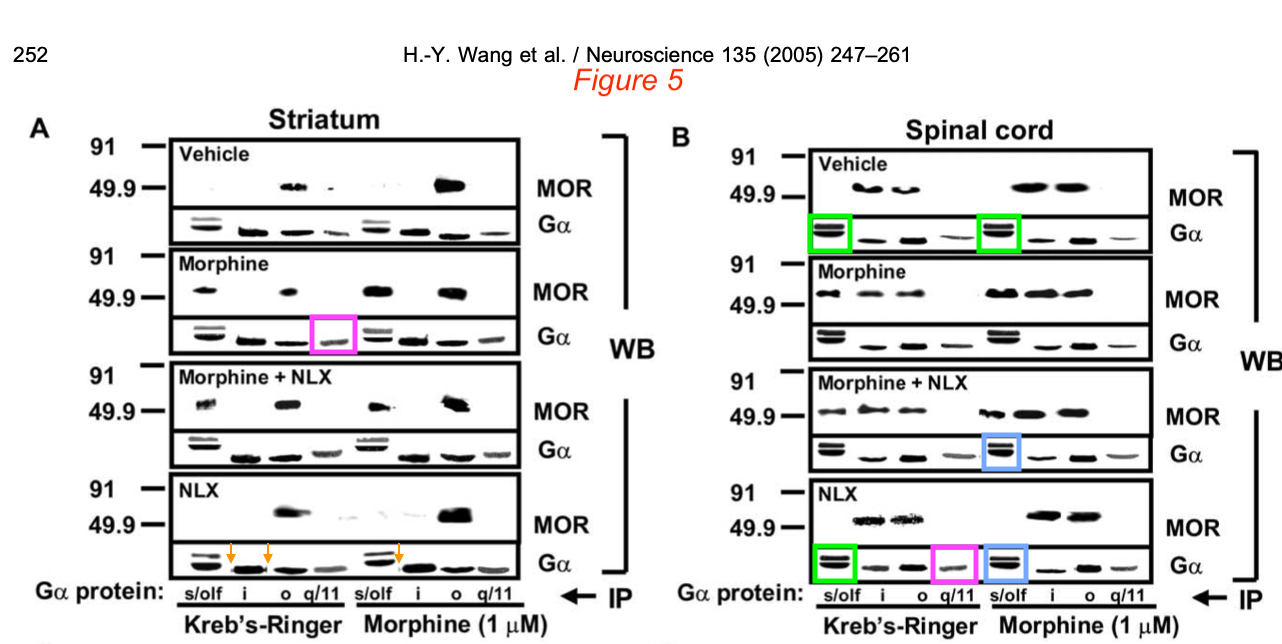
Then there were even more duplicated gel bands. Where the Labaton Sucharow letter reported “Telltale signs that the Gα bands in Figure 5a likely come from different gels“, Bik followed up on the “Additional Suspicious Western Blots” in Section E and found:
- “Blue and green boxes: It was noted in the report mentioned above that some of the “double decker” bands look similar to each other. The resolution is low, but maybe the authors could take away these concerns by showing the uncropped blots, if they still are available?
- Pink boxes: An additional concern not noted in the report is that two of the G-alpha bands, representing seemingly different experiments (Morphine vs NLX), look quite similar, while their surrounding bands are very different.
- Orange arrows: some artifacts around bands suggestive of splicing.“
That paper doesn’t have a conflict of interest section, apparently back in 2005 this concept was not invented by Elsevier yet.
As mentioned above, that Wang et al 2005 paper contained data later reused in Wang et al 2010:
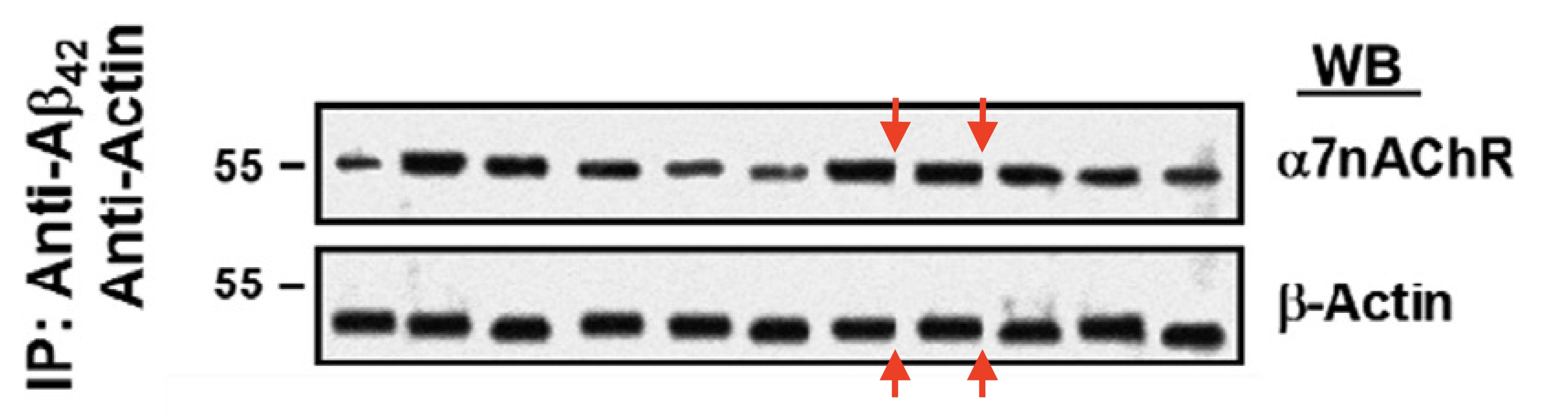
Hoau-Yan Wang, Kalindi Bakshi, Changpeng Shen , Maya Frankfurt, Caryn Trocmé-Thibierge, Philippe Morain S 24795 limits beta-amyloid-alpha7 nicotinic receptor interaction and reduces Alzheimer’s disease-like pathologies Biological Psychiatry (2010) doi: 10.1016/j.biopsych.2009.09.031
That study with a proprietary drug was funded by another pharma company, Servier, which also paid Wang as consultant. Bik found that paper was also falsified:
“Figure 1A appears to show a lighter background around some, but not all bands in the top a7nAChR blot. A similar lighter area is visible around all bands in the lower blot.“
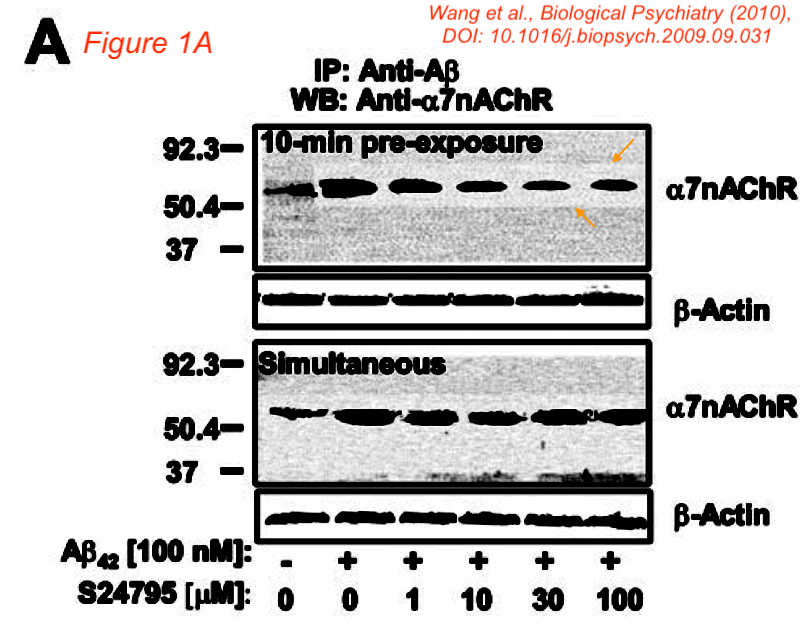
Such things happen when a gel image gets assembled or otherwise modified in Photoshop, with the purpose of data forgery.
Moving on to the next paper:
Hoau-Yan Wang, Maya Frankfurt , Lindsay H. Burns High-Affinity Naloxone Binding to Filamin A Prevents Mu Opioid Receptor–Gs Coupling Underlying Opioid Tolerance and Dependence PLoS ONE (2008) doi: 10.1371/journal.pone.0001554
The paper mentions as conflict of interests that “This study was supported by Pain Therapeutics, Inc. and LHB is an employee of this company“, but Wang forgot to mention that he was paid by Pain Therapeutics (now Cassava) as advisory board member.
The lawyer’s report criticised gel splicing and added “Figure 7a of that paper appears to show two IDENTICAL panels (red arrows) for what are reported as different experiments.”
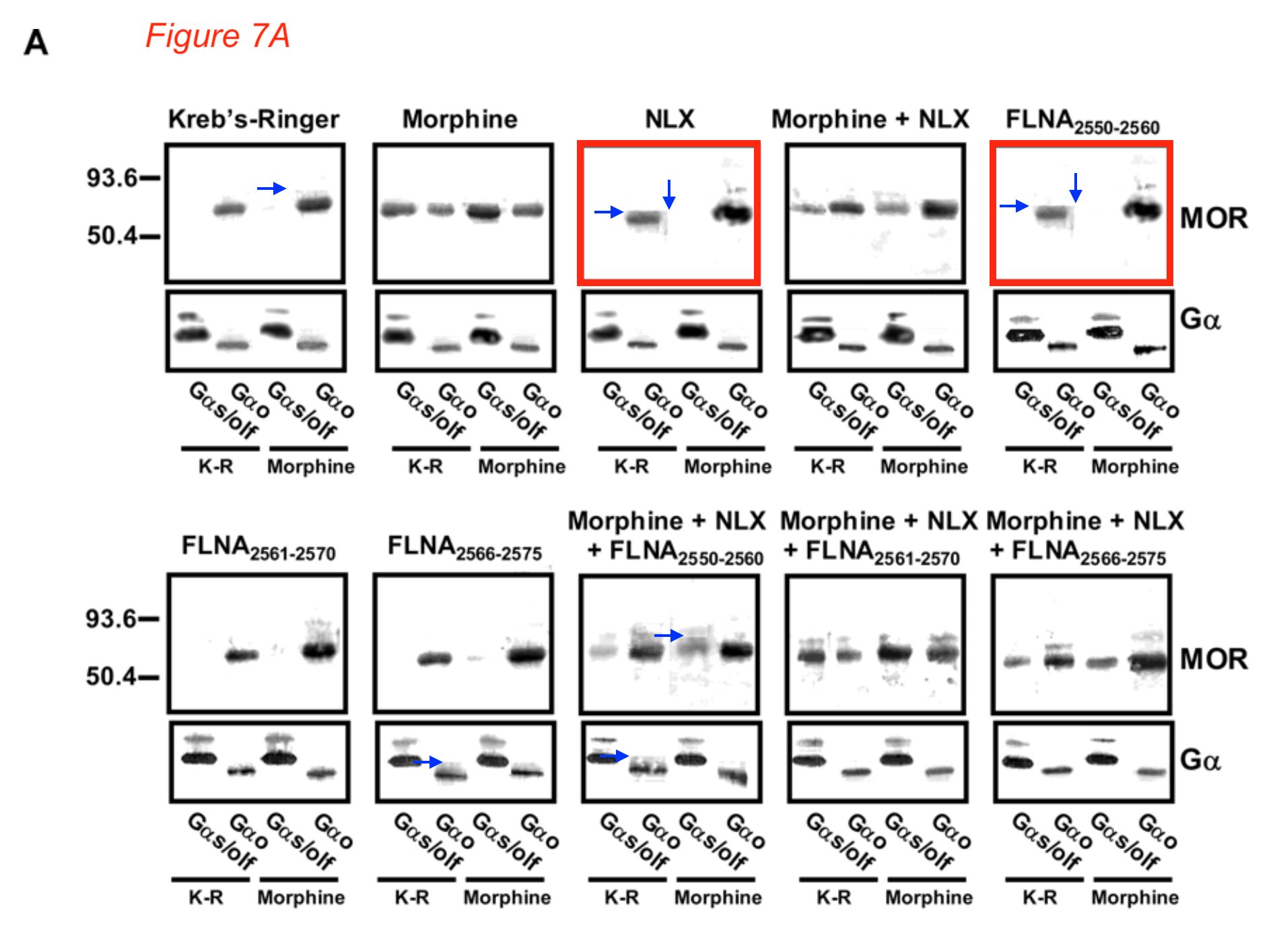
Blue arrows: Some sharp background transitions around bands, suggestive of splicing or patching.“
Cassava rebutted such evidence with this statement:
“Fiction: Cassava Sciences’ Western blots data appear overexposed and highly processed, evidence of image manipulation.
Fact: High quality bands are supposed to look sharp6. Smudged bands can be evidence of inexperience, depending on levels of protein in the band.“
I love this. If you gels lack sharp edges around the bands, it’s because you are an inexperienced loser. This pigheaded arrogance is breathtaking, do they really think everyone else is as scientifically illiterate as they are?
The next discussed paper about curing Alzheimer’s with Filamin A was this:
Hoau-Yan Wang , Kalindi Bakshi , Maya Frankfurt , Andres Stucky , Marissa Goberdhan , Sanket M Shah , Lindsay H Burns Reducing amyloid-related Alzheimer’s disease pathogenesis by a small molecule targeting filamin A The Journal of neuroscience (2012) doi: 10.1523/jneurosci.0354-12.2012
At least here, conflicts of interests were declared: “L.H.B. is an employee of and H.-Y.W. is a consultant for Pain Therapeutics, Inc.“. But not the massive fraud in the presented data!
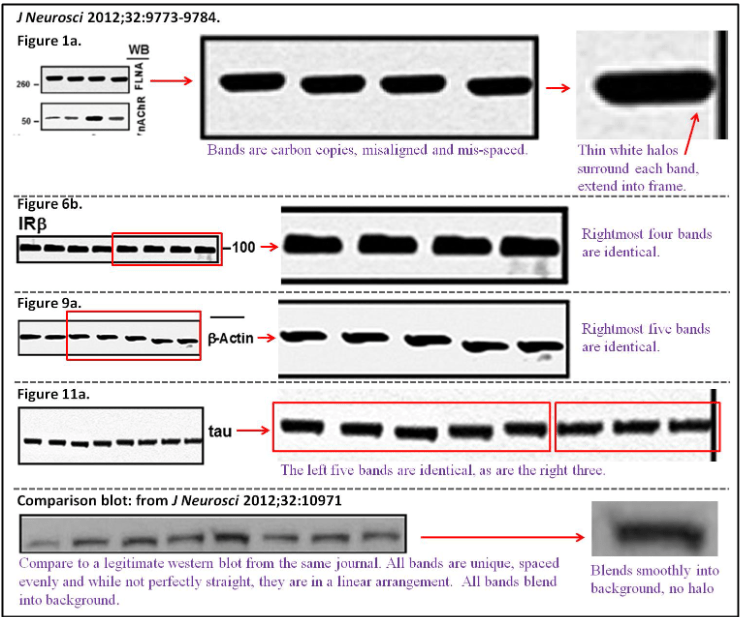
The Labaton Sucharow letter explains:

“In Figure 1a, the four Filamin A bands in the top set are more similar to each than can be expected by chance and appear to be duplicates. The images at right are magnified, showing that the pixels containing the bands are essentially identical. Additionally, the blots are not aligned and the spacing is irregular. Because FLNA is a large protein (~290kDa), it does not migrate in the gel very far; therefore, this degree of misalignment is suspicious. Moreover, the thin white halos surrounding each band are concerning. There are optical reasons why a halo (or ringing artifact) could occur, but this artifact is most common when components from multiple images are combined using photo editing software. This halo artifact is more prominent in the questionable blots, and extends in some cases into the frame around the blot which is hard to explain as an
optical phenomenon.“
This was Cassava’s retort:
“Fiction: “Halo” effects in certain bands indicate fraud.
Fact: A “Halo” effects in certain bands is a direct result of very dense dark loading control bands.7Fiction: Unusual looking bands on Western blots were pieced together from multiple sources.
Fact: Proteins can and do stick to the side of a lane and migrate that way, resulting in ‘candy-wrapper’ appearance or other fictional images.“
It wasn’t the “loading control bands” which have a halo effect, even outside the gel. And the “‘candy-wrapper’ appearance” derives from digital gel band splicing, where some kack-handed fraudster accidentally cuts into neighbouring bands. But yes, I agree, the images presented in those papers are “fictional“.
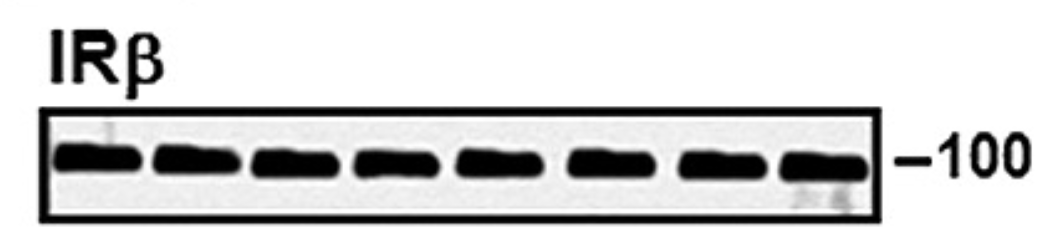
Bik found more, like the completely fake western blots of IRbeta in Figures 6A and 6B, which were most obviously assembled in Photoshop, sometimes from copy-pasted bands:
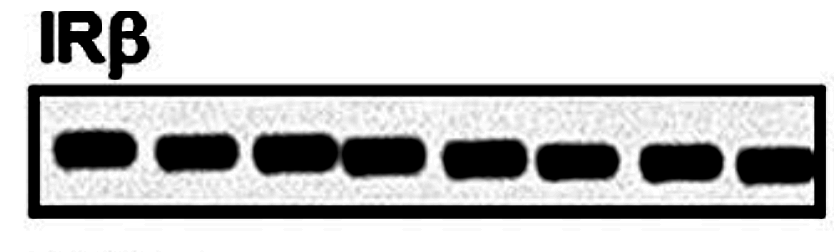
Figures 9A and 11 A proved to be fraudulent disasters also:

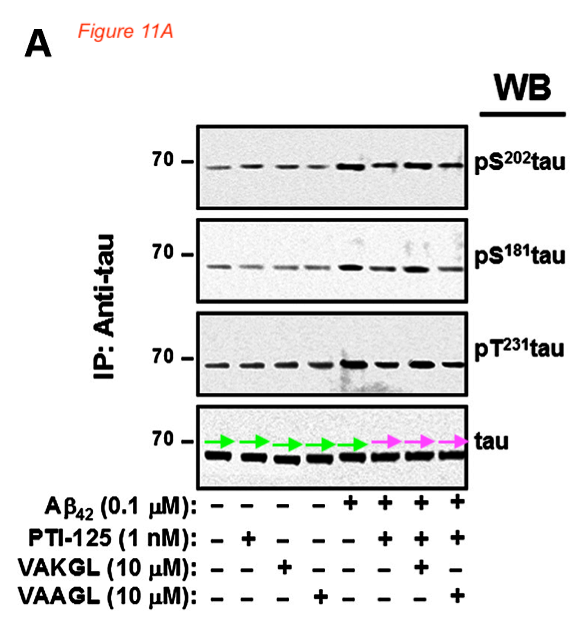
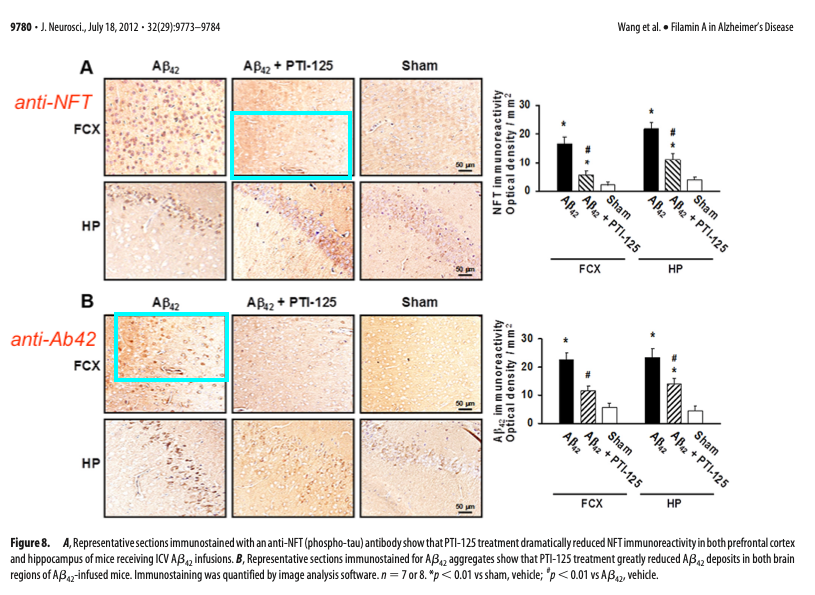
Those above are fake western blots, made with one single band copy-pasted several times, as the arrows highlight. To add some more colours to that misery, Bik found a recycled immunohistochemistry image, to stand in for different antibody stainings of different experiments.
On to the last paper criticised in the lawyer’s letter to FDA!
Hoau-Yan Wang, Kuo-Chieh Lee , Zhe Pei , Amber Khan , Kalindi Bakshi, Lindsay H. Burns PTI-125 binds and reverses an altered conformation of filamin A to reduce Alzheimer’s disease pathogenesis Neurobiology of Aging (2017) doi: 10.1016/j.neurobiolaging.2017.03.016
The Figure 12 of that Wang et al 2017 paper contains a skilfully falsified western blot. Impressive is that Cassava sees no problem with same gel having either 12 lanes or 13, depending which protein is shown:

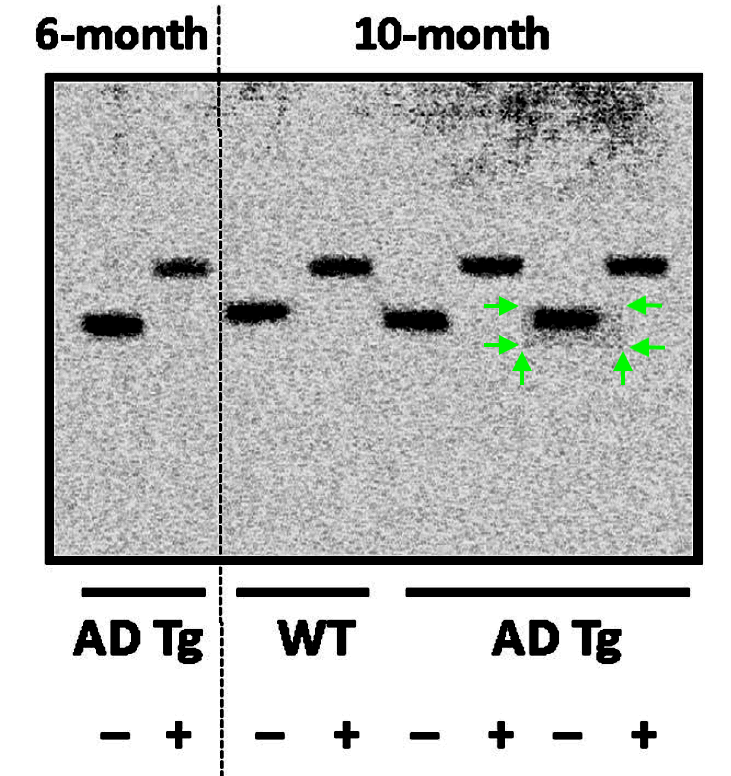
In Figure 3, Bik reported more:
“One of the bands representing a 10-month sample, in the right blot, appears to be surrounded by a rectangle of a different background than the rest of the blot.“
That is because that gel band was digitally inserted in Photoshop, and/or selectively modified inside the rectangle area.
Bik then found similar issues in Figure 10B, gel bands digitally inserted, likely to once again to replace undesired real results:
Also this study’s mouse behavioural data in the so-called Y-Maze experiments was questioned, because “if the result were legitimate, the drug treatment changing the mice’s behavior to closer to 50% spontaneous alternation (i.e., closer to random) would be more accurately interpreted as evidence of worse memory performance.“
Cassava reacted with:
“Fiction: Changes in the Y-maze test for transgenic mice could be interpreted as a decline in cognition.
Fact: A panel of independent, peer-reviewers believe these changes represent an improvement, along with significant improvements in two other behavior tests.“
Which basically means, haha, it doesn’t matter if our results make sense, they passed peer review so you are legally not allowed to criticise them anymore. I guess same applies to the Photoshop fabrications?
Indeed, that paper, as well as the above Wang J Neuroscience 2021 plus Wang et al J Neuroscience 2009, all passed peer review, despite being biologically improbable, as the Labaton Sucharow letter explains:
“In one key line of experiments, the authors report that this entire mechanism can be observed in post-mortem human brain tissue from subjects with
Alzheimer’s disease and neurological controls. […] In these experiments, post-mortem human brain tissue is warmed from -80°C to -20°C and chopped into 200micron x 200micron x 3mm blocks with a McIlwain chopper (as a side
note, a McIlwain chopper doesn’t effectively cut frozen tissue). The resulting chopped tissue is treated with β-amyloid and the experimental drug for 1 hour. They then report a massive increase in tau phosphorylation (modification of the tau protein by enzymatic addition of a phosphate group to the protein; up to 10 fold) from β-amyloid treatment in untreated samples; and that tau phosphorylation was blocked by addition of PTI-125. It is unlikely that the enzyme
responsible for phosphorylation would survive the initial -80°C freezing step. Moreover, the phosphorylation experiments are reported to have been performed at 4°C, but it is unlikely that the enzyme responsible for phosphorylation would be active at 4°C (enzymes generally work best at body temperature—37°C)“
Indeed, valid points, which suggest either that the peer reviewers were either not reading the paper they approved, or were incompetent and scientifically illiterate themselves. But the situation was even worse:
“The methodology for the post-mortem human brain experiments among the three studies are virtually word-for-word identical. The age and post-mortem interval for the groups of subjects are the same (down to the decimal points) in each of the three papers. It is therefore reasonable to assume the same human brain specimens were used across the studies from 2008-2017, so the results are premised on the enzymes in the human brain extracts remaining active up to 13 hours post-mortem before freezing, remaining active after nearly 10 years in frozen archival without any advanced cryopreservative techniques, and being active at 4°C.”
The company retorted with:
“Cassava Sciences is not aware of an industry-wide ‘expiration date’ on human post-mortem brain tissue that is properly collected, processed and stored.“
I wish they would instead explain how they made the native brain enzymes work under such conditions though.
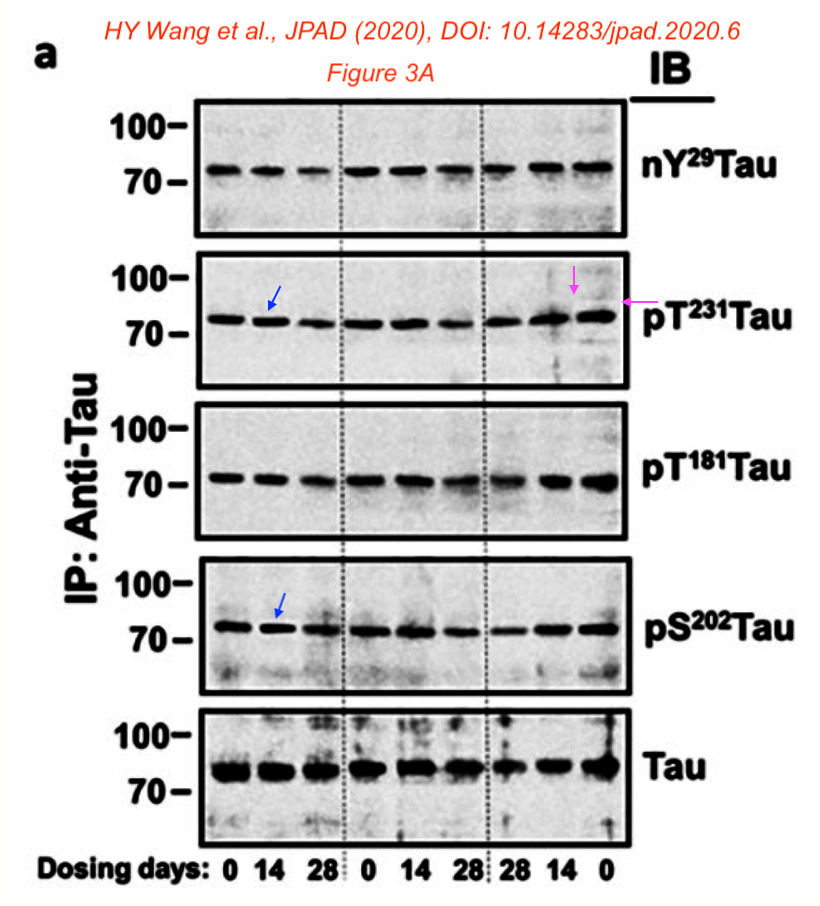
Those were not the only problematic papers by Wang. Here one with the company’s founder and CEO Remi Barbier and the Chief Medical Officer Nadav Friedmann as co-authors:
H.-Y. Wang, Z. Pei , K.-C. Lee , E. Lopez-Brignoni , B. Nikolov , C.A. Crowley , M.R. Marsman , R. Barbier , N. Friedmann, L.H. Burns PTI-125 REDUCES BIOMARKERS OF ALZHEIMER’S DISEASE IN PATIENTS The Journal of Prevention of Alzheimer s Disease (2020) doi: 10.14283/jpad.2020.6
Bik criticised that the paper was peer reviewed in just 6 days. And she found a problematic gel in Figure 3A:
- “Slanted blue arrows: Some bands appear to float over the background, surrounded by a white halo, and with a sharper appearance than other bands
- Pink arrows: The right-most band in the pT231Tau lane appears to be surrounded by a rectangular box with a different background appearance as the other lanes.”
Jay J. Paquette , Hoau-Yan Wang, Kalindi Bakshi, Mary C. Olmstead Cannabinoid-induced tolerance is associated with a CB1 receptor G protein coupling switch that is prevented by ultra-low dose rimonabant Behavioural Pharmacology (2007) doi: 10.1097/fbp.0b013e3282f15890

Bik found problems with Figure 4A.
- “Orange boxes: Two of the double decker G alpha bands appear to look similar, if one of them is flipped horizontally. Note a slanted stripe in the middle of the top band.
- Green boxes: Two other double decker bands in the bottom left blot look similar
- Teal boxes: Two single bands in different G alpha blots look similar“
Presumably, if your western blots lack duplicated bands, it is the sign of your “inexperience”.
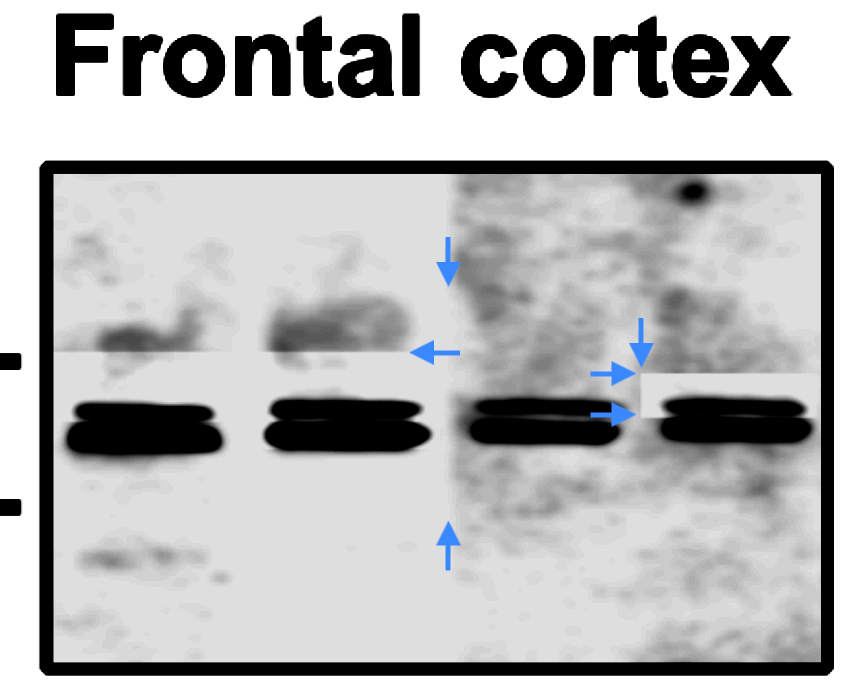
Kalindi Bakshi, Raminder Parihar, Satindra K. Goswami , Melissa Walsh , Eitan Friedman, Hoau-Yan Wang Prenatal cocaine exposure uncouples mGluR1 from Homer1 and Gq Proteins PLoS ONE (2014) doi: 10.1371/journal.pone.0091671
Bik found two figures very skilfully falsified. For example, Figure 2A.
“In the Homer 1 / Frontal cortex blot, unexpected sharp horizontal and vertical background transitions appear to be visible. Of particular note, the top band in the right-most lane appears surrounded by a rectangular box of a different background. Shown with blue arrows.“

Finally, one very new Wang paper (The authors declare no competing financial interests) was flagged by an anonymous PubPeer commenter:
Gavin M. Meade , Lily S. Charron , Lantz W. Kilburn , Zhe Pei , Hoau-Yan Wang , Siobhan Robinson A model of negative emotional contagion between male-female rat dyads: Effects of voluntary exercise on stress-induced behavior and BDNF-TrkB signaling Physiology & Behavior (2021) doi: 10.1016/j.physbeh.2020.113286
There are similar issues with Figures 7b, 8a, and 9a.”
Section E of the lawyer’s letter to FDA is about “Six Additional Areas of Concern“, discussing “suspicious” scientific claims and flagging “Additional Suspicious Western Blots“, the latter largely confirmed by Bik.
Part D asks FDA to suspend Cassava’s scheduled phase 3 clinical trial NCT04994483 with 750 participants, and to audit all past clincial trials this company performed. NIH and the New York University are asked to investigate Wang’s research, the journals are invited to retract the falsified papers.
The Labaton Sucharow lawyers also discuss irregularities in the trial data, like this:
“it seems that the primary biomarker data set we have with simufilam in Alzheimer’s disease that was entirely produced and finalized by an external lab found that the drug had no effect on clinical biomarkers. Cassava replaced this with a reanalysis that was finalized by an academic lab (presumably Dr.
Wang) and showed that simufilam showed remarkable benefit. Second, plasma biomarker data from these same patients, which were just presented by Cassava Sciences, contains evidence of manipulation. If there’s no biomarker signal, and there is apparent misrepresentation of clinical data the continuation of the ongoing Cassava trials may put patients at risk without the claimed evidence of biomarker benefit.”
Cassava’s reply to it sounds like what Chinese cheaters post on PubPeer blaming an “external company”:
“Fiction: Biomarker data is generated by Cassava Sciences or its science collaborators and therefore are falsified.
Fact: Cassava Sciences’ plasma p-tau data from Alzheimer’s patients was generated by Quanterix Corp., an independent company, and presented at the recent Alzheimer’s Association International Conference1.“
Problem is, Quanterix was not prepared to play the scapegoat and was quoted:
“In Friday’s statement, Quanterix said it is “widely recognized for its commitment to business integrity and to upholding the highest standards of quality.” Quanterix’s stock rose 4.5% in premarket trading, while Cassava shares tumbled 21.2%”
Apparently unconvinced by Cassava’s explanations, The City College of New York opened a research misconduct investigation of Wang’s papers. Tony M. Liss, Provost and Senior Vice President for Academic Affairs, confirmed it to me:
“The College takes accusations of research misconduct very seriously. When such an accusation is made the College Research Integrity Officer follows the CUNY Policy Regarding the Disposition of Allegations of Research Misconduct . Until that process is completed, we are unable to comment or respond to inquiries.“
Maybe this is why Cassava started to distance themselves from Wang in their next statement from 27.08.2021:
“No, the preclinical and clinical foundations linking Filamin A to Alzheimer’s disease do not derive only from the publications of Drs. Wang and Burns.“
As if that proves anything. The Seeking Alpha article (allegedly written by a “Joe Springer”, if you believe that) lists several research papers on Filamin A (who gave those to Joe?), and then warns: “Trials are only halted for safety, and simufilam is shown safer than placebo, there is no chance the FDA will act on the petition’s requests.“
Apparently they think research fraud is not a reason to suspend a clinical trial.
The above mentioned Athira Pharma placed their own founding CEO on leave after Leen Kawas’ papers were reported for data manipulation. But Cassava Sciences keeps lashing out at critics instead of dismissing Wang and Burns. This pharma startup seems to be at best utterly ignorant how science is done properly, and at worst, well, you saw the gel pictures and Cassava’s press releases. Not really reassuring to the investors, no wonder that the stocks are in freefall. What will happen to the clinical trials now?
Update 29./30.08.2021
On 29 July 2021, Cassava proudly announced interim results of an open-label clinical trial with Simufilam (PTI-125) with 150 participants. The trial is described to have started in March 2020, and was not only unblinded, but it also didn’t have a control arm (which makes that whole trial rather pointless).
The description of the NCT04388254 trial, which somehow managed to be Open-Label (unblinded) and quadruply blinded, as well as both with and without control arm, is confusing at best (hence my correction of the previous update):
“Approximately two hundred (200) patients will be enrolled into the study. All participants will receive open-label simufilam 100 mg b.i.d. for a year. At Month12, participants will be randomized (1:1) to continue taking simufilam 100 mg b.i.d. or to be switched to placebo for 6 months. At Month 18, all participants will enter a final 6-month treatment period of open-label simufilam 100 mg b.i.d.“
The switch from “A 12-Month, Open-Label Safety Study of PTI-125” to “Simufilam Followed by a 6-Month Randomized Withdrawal and 6 Additional Months Open-Label” happened on 30 July 2020. Also, the number of patients to be recruited jumped from 100 to 200, but now the press release changed it to 150: “The open-label study has completed its target enrollment of 150 subjects“. It all looks like an insane trick to somehow turn something extremely biased and unreliable into a gold standard of a blinded randomised controlled clinical trial.
The now announced open-label trial was founded by NIH in 2020 (grant #1R44AG065152-01) as a continuation of the previous open-label trial by Cassava NCT03748706, which took place March-May 2019 and treated 13 patients. Its results were published by our trustworthy friends Wang and Burns (the latter is listed as principal investigator of both the old and the new open-label trial):
Wang HY, Pei Z, Lee KC, Lopez-Brignoni E, Nikolov B, Crowley CA, Marsman MR, Barbier R, Friedmann N, Burns LH. PTI-125 Reduces Biomarkers of Alzheimer’s Disease in Patients. J Prev Alzheimers Dis. (2020). doi: 10.14283/jpad.2020.6
I apologise for the confusion in the update before, I never saw a quadruple-blinded but open-label while one-arm but placebo-controlled trial with 150 or maybe 200 participants before 😉
Update 30.08.2021
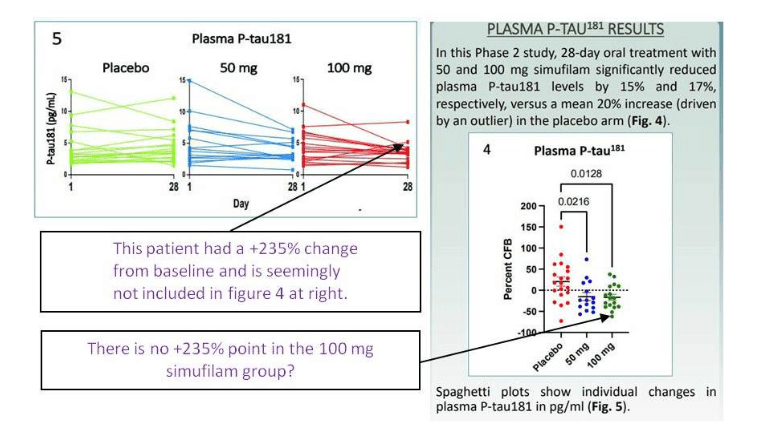
Elisabeth Bik wrote a follow up, and she found several inconsistencies in the Cassava clinical data from the contested phase 2 trial NCT04079803 and its results presented as poster Wang et al at a conference in July 2021. Bik notes:

“The poster states that 64 patients were included in the study and randomly assigned to three groups. There are no details given about how many patients were assigned to each group, but we would expect roughly 20 people per group. Yet, Figure 4 only shows 20, 15, and 17 data points for the placebo, 50 mg, and 100 mg Simufilam groups, respectively. That is a total of 52 patients. What happened to the 12 other patients?“
Well, maybe these patients were all misbehaving outliers, you know… Look, two of them left the placebo group eventually, one disappeared, the other felt so well that participant joined the treatment group:
“While Figure 4 shows 20/15/17 (sum=52) data points for the three groups, Figure 5 appears to show 18/15/18 data points (sum=51). The numbers of the placebo and 100 mg treatment groups therefore do not match.“
Actually, the patients walked in both directions. Those whose Alzheimer’s decease worsened, got up and joined the placebo group:
“Of particular note, in Figure 5, the P-tau181 value from a patient in the 100 mg treatment group went up from ~2.1 to ~5.2, which is an increase of 150%. In other words, one of the patients in the 100 mg treatment group showed a large increase of the Alzheimer’s disease biomarker . Yet, this particular data point is missing in the 100 mg treatment group in Figure 4. Instead, the 150% increase data point is included in the placebo group.“
Watching all that pathetic data fudgery is PAINful, pun intended.
A reader pointed me to this preprint with same trial results:
Hoau-Yan Wang, Zhe Pei, Kuo-Chieh Lee, Yaneicy Gonzalez Rojas, Tamara Doehner, John Puente, Patrick Sciara, Brian Beck, Evelyn Lopez-Brignoni, Boris Nikolov, Carrie Crowley, George Ben Thornton, Remi Barbier, Nadav Friedmann, Jeffrey L. Cummings, Lindsay Burns Effects of simufilam on cerebrospinal fluid biomarkers in Alzheimer’s disease: A randomized clinical trial ResearchSquare (2021) DOI: 10.21203/rs.3.rs-249858/v1
Update 4.09.2021
According to this source (apparently based on this paywalled STAT News article), Remi Barbier and Lindsay Burns are married. So now we know why Barbier keeps defending the obvious Photoshop forgeries his wife published with Wang:
“I am aware that the allegations have caused a storm of opinions and counter-opinions on the internet around the fine visual features of photograph of certain Western blots. We all know that once a photograph is on the internet, the pixels that make up that photograph can easily be Photoshopped, cropped or otherwise distorted to mean anything you want it to mean.
Furthermore, internet photos are resolution dependent. This means an internet photo can quickly lose quality and look blurry or pixelated, or whatever. I don’t trust the authenticity of photos on the internet, and neither should you.“
It must be a global conspiracy against Cassava which involves everyone in scholarly publishing and academia, going back many years already?
Update 10.11.2021
On 4 November 2021, Cassava issued a press release regarding the disastrous paper paper Wang et al J Neuroscience 2012 (discussed above):
“The Journal of Neuroscience authorized Cassava Sciences to share a statement on this matter, reprinted in full below:
“The Journal of Neuroscience follows COPE [ C ommittee o n P ublication E thics] guidelines and takes any claims of misconduct very seriously. In response to allegations of data manipulation in JNeurosci 2012;32:9773-9784 the Journal requested raw data, including images of original, uncropped Western blots. The Journal determined that there was one duplicated panel in Figure 8 and a Corrigendum was requested and will be printed. No evidence of data manipulation was found for Western blot data.”“
I was very curious about those mysterious uncropped blots. Pity the journal’s Editor-in-Chief Marina Picciotto, professor of neuroscience in Yale, ignored all my emails.
Now, on 10 November 2021, the Erratum appeared:
“The original, uncropped blots of loading control bands in Figure 6, Aand B, are shown below.
The original, uncropped blots for b-actin in Figure 9Aare also shown below. The left image is the higher-resolution image with the additional bands cropped out, as seen in the full image on the right.“
Here are those “original, uncropped blots”, lo and behold (I merely boosted the levels):
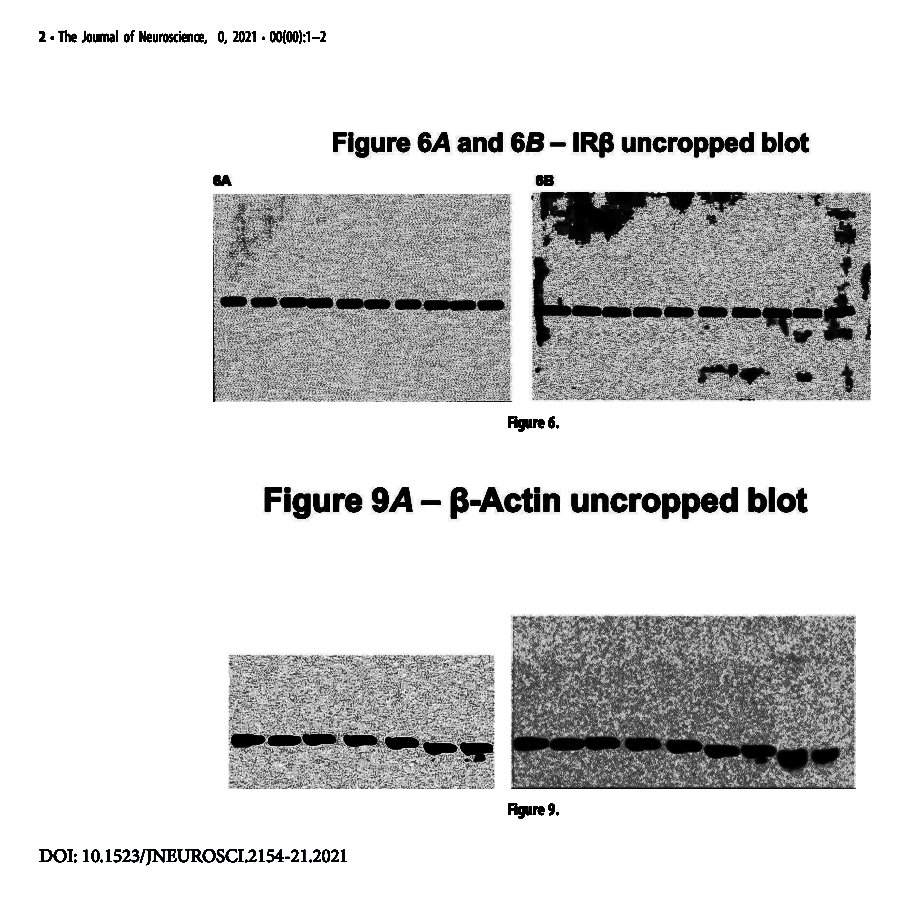
Now, Dr Picciotto and her editorial colleagues are surely the real experts, but what the actual f*** are these pictures, really?
Observe that the gel bands go from one edge of the membrane to another. Meaning, if those were real gels (not likely):
- for the two top gels with 10 lanes each there never was any marker lane loaded. Do I really need to explain to DOCTOR Picciotto why this is important?
- for the bottom gels (“uncropped” on the right) not only there is no marker lane but they even cut the membrane to blot exactly only the 9 gel lanes they loaded and not the last, empty lane where they could have loaded a marker. Also, what is that shadow which accidentally exactly fits the cropped version of that same gel on bottom left (also spotted by Bik)? The background pattern is also different between cropped and uncropped actin.
But if our Yale professor says this makes sense, who is the failed scientist here?

Update 17.11.2021
Cassava made it into The Wall Street Journal, because the company with “the sixth-best performing U.S. stock this year“, once valued at $5 Billion, is now under investigation by the Securities and Exchange Commission (SEC), the US government’s stockmarket watchdog. Cassava is also under investigation by National Institutes of Health (NIH), who may want to recover their $20 million grant money.
We now also know who submitted that FDA letter:
“The petition’s authors are two physicians who said they came to doubt Cassava’s research and have shorted its stock, betting the share price would fall once investors recognized the problems they found, they said. David Bredt, a biotech entrepreneur and former neuroscience research chief at Johnson & Johnson and Eli Lilly & Co., and Geoffrey Pitt, a cardiologist and professor at Weill Cornell Medicine, wrote that Cassava’s research, published in several different scientific journals, include images of experiments that appear to have been manipulated using software such as Photoshop.”
Update 21.12.2021
There is a follow-up article, with even more Cassava fraud, and even more embarrassment for regulatory authorities and scientific journals!
Cassava Sciences never replied to my emails when asked to comment about the growing PubPeer evidence or the clinical trial registration issues.

Get For Better Science delivered to your inbox.
Make a one-time donation
Make a monthly donation
Choose an amount
Or enter a custom amount
Your contribution is appreciated.
Your contribution is appreciated.
DonateDonate monthly

Simuflimflam.
LikeLike
A tour de force. Congrats.
LikeLike
In order to allege that this is fraud you first have to assert that the peer reviewers, the NIH, and the FDA all whiffed, not just on one test, but on multiple tests. AND they have access to the actual data in a more involved way than the public.
Second, the phase 2 study and the open label are supportive of the drug. To say SAVA is a fraud based on this would be like saying a Major League Baseball player who has been playing pro for a few years is a fraud based on a possible faulty test in college.
The obsession around small details is obscuring the fact that SAVA proceeded through to phase 3 with experts in the NIH and FDA collaborating closely. The CEO did not sell despite a 7000% increase in his shares. And Adam Feuerstein has publicly copped to having a personal vendetta against the CEO due to his involvement with Remoxy.
Good luck with your short. A citizens petition is well known to the FDA as a weapon, not a fact finding mission, and that it was filed for a drug that isn’t even past Phase 3 AND by a party which disclosed a short position means this filing goes in the trash.
LikeLike
Jack, maybe you commented under the wrong article on the wrong website (“Baseball”?), but here we were discussing western blots. Nobody but the papers’ authors had access to the raw data, and CUNY is currently trying to find out if that raw data ever exited as such. Chill, Jack.
LikeLike
You are dodging my point. A supposed fraudulent ink blot test wouldn’t somehow translate into a drug that passed phase 2 and is corroborating its premise with an ongoing open label. You want to imply the company is dirty but can’t address anything of substance regarding the drug.
LikeLike
Jack, I don’t know what an ink blot test is, but I’m sure you will educate us any moment. Or do you mean Rorschach?
The phase 2 trial data is being disputed in a mud-slinging match between two biotech companies right now.
The recruiting Phase 3 trial is not “open label”, I think you erroneously used that term because it sounds so sciency to you?
https://clinicaltrials.gov/ct2/show/NCT04388254
LikeLike
They currently have an open label trial going on right now. The 12 month data is due any day now. You know nothing about the company you are attempting to undermine. It is not the same as the phase 3.
LikeLike
I found this open-label (unblinded) trial!
https://clinicaltrials.gov/ct2/show/NCT03748706
It had 13 patients and no control arm, started in March 2019, and ended in May 2019. Results proudly announced only now:
https://www.cassavasciences.com/news-releases/news-release-details/cassava-sciences-announces-positive-cognition-data-simufilam
Now, you are obviously the expert here, but generally such kind of tiny-scale uncontrolled unblinded trials is done by investigators who are afraid of results they would get from proper RCTs. Like the disputed phase 2 data now.
Interesting are these new claims:
“About the Open-label Study
In March 2020, Cassava Sciences initiated a long-term, open-label study to evaluate simufilam in patients with Alzheimer’s disease. This study is funded by a research grant award from the National Institutes of Health (NIH). The open-label study is intended to monitor the long-term safety and tolerability of simufilam 100 mg twice-daily for 12 months or longer in patients with Alzheimer’s disease. Another study objective is to measure changes in cognition on ADAS-Cog, a standard test of cognition in Alzheimer’s disease. The study protocol has pre-specified interim analyses on safety and cognition for the first 50 subjects who complete 6, 9 and 12 months of drug treatment. The study protocol also specifies two biomarker measurements: i) from baseline to Month 6 in 25 study subjects, and ii) baseline to Month 12 in another 25 study subjects. The open-label study has completed its target enrollment of 150 subjects. By physician and patient request, clinical sites may continue to enroll additional subjects up through the upcoming initiation of the Company’s Phase 3 pivotal program of simufilam.”
So not only was the trial shifted by a a whole year, it now has whopping 150 patients instead of original 13. Last changes to registration were made in June 2021, and the authors still insisted it was just 13 patients treated in early 2019.
Which is not acceptable practice in medicine and makes the results even less trustworthy.
Finally, look, who published the results, same people who published fake preclinical data:
Wang HY, Pei Z, Lee KC, Lopez-Brignoni E, Nikolov B, Crowley CA, Marsman MR, Barbier R, Friedmann N, Burns LH. PTI-125 Reduces Biomarkers of Alzheimer’s Disease in Patients. J Prev Alzheimers Dis. 2020;7(4):256-264. doi: 10.14283/jpad.2020.6.
It had 13 patients as of November 2019
“Patients
Of 19 patients screened, 13 enrolled. All 13 were mild-to-moderate AD patients, age 50-85, MMSE ≥ 16 and ≤ 24, with a CSF T-tau/Aβ42 ratio ≥ 0.30. Although the protocol stated 12 patients would be enrolled, 13 were enrolled due to multiple patients in screening near the end of the study.”
LikeLike
Listing a study does not mean it has been evaluated by the U.S. Federal Government. NIH provided research funds, not input to the trial. FDA not involved here at all. Reviewers of originating papers likely buddies of authors and chimps. Anybody can set up a clinical trial in the US, provided you get proper consents from subjects desperate enough to participate. There is no host institution, and participant accrual appears to have been very weak until the company started pimping “promising results” from their tiny and no doubt cherry-picked data set. That will bring in the desperate punters. This is quite a shameful exercise from all angles.
LikeLike
Claiming that the Company made statements that are damaging and then providing a link to a Seeking Alpha article and that person’s opinions (not the Company’s statements) is not exactly presenting truthful facts.
LikeLike
You have too much admiration for Joe Springer. But unlike he claims, it’s unlikely he wrote this press release text, but merely posted it under his name. Common practice in such circles.
https://seekingalpha.com/article/4452217-specious-claims-spurious-accusations-against-cassava-sciences
Made a long list of allegedly relevant research papers, all by himself, that’s how educated in biomedical sciences our Joe is?
He also forgot to change the plural “we” to “I”:
“We think the petition was all smoke and mirrors and will go nowhere at all, but it was obviously effective in clobbering the share price (again).[…]
We also think it is quite bullish that the powers that be are pulling out all of the stops to slander and sabotage this company and drug, and there is virtually nothing of substance they can come up with.”
Go chase journalistic malpractices where they really happen, Ashton.
LikeLike
Leonid, your article states as a fact that the Company made those statements. They did not. Joe Springer made those opinions. I guess in your reply you assume the Company planted those statements with a random seeking alpha writer? Why would you claim the company made them? intentional manipulation or honest mistake? Either way, you lost credibility by doing that as a “journalist.”
LikeLike
Unlike Joe Springer, I don’t have any credibility as “journalist”, Ashton.
But for legal reasons, I changed the text a bit. For all I care, Joe Springer can continue insisting he assembled that list of research papers all by himself.
LikeLike
Ha Ha, Leonid, How much that shorty indicates paid you to write you these all old rubbish? OR you are the one of members of that gang?
LikeLike
I work for Gilead, Tom. Check Twitter.
LikeLike
Seems odd that nobody involved in this story bothered to ask: how can a drug that does not cross the blood-brain barrier affect an organ that has almost no FLNA? Platelets, which are abundant in the bloodstream, are chock full of it. What a bio-failure.
LikeLike
Ha! Our biomed genius Joe Springer (whose article was never Cassava-ghostwritten, I am educated) has assembled a whole list of FLNA papers here:
https://seekingalpha.com/article/4452217-specious-claims-spurious-accusations-against-cassava-sciences
LikeLike
Owlbert, I think you misunderstand the situation. The drug is a small molecule so it does pass the blood brain barrier. Look up small molecule on Wikipedia.
Also, The open label study was done primarily for safety reasons. But, I believe the approach to take additional adas-cog cognition tests as well as blood and csf spinal tests makes sense to help guide future testing like the phase 3 which is about to start. It also provides a good baseline to compare the phase 3 data to as it comes in. As the medical community knows, the final results of the OLS are helpful and supportive, but by themselves insufficient to draw definitive conclusions regarding efficacy. But that does not mean the study is flawed or worthless, only that the results of it need context.
LikeLike
I love how guys who invested in Cassava now explain Owlbert how biology and medicine work. Your “small molecule” explanation is hilarious!
LikeLike
You might want to look up blood-brain barrier on Wikipedia as well. Does not pass the vast majority of small lipophilic molecules, which is why getting past it is a major challenge in drug design. The molecule in question here has never been described beyond mention of it being about 300D. No pharmacokinetic data reported, and all the papers involving it appear to be riddled with fraud. The “clinical trial” lodged with the US government website proposed to give everyone the drug for a year, then create a double-blind drug/placebo trial for six months, then shift to open label. Readouts are all over the shop, and all aspects of the study are highly susceptible to confirmation bias. There will not be an honest dime made from this, but some lucky dodger may cash in. I just wonder who is paying for all this?
LikeLike
Another flag is that these papers pulled their pharmacokinetics out of someone’s – or some rodent’s – fundamental orifice. A <300D mystery molecule that works IP and orally, with no side-effects at doses up to 2g/kg body weight (in rats). Anybody who gave these guys a nickel deserves to lose a dollar.
LikeLike
Oh, don’t say that. I just bought on the dip last week betting that your favorite biomed genius could quickly pump the stock price back up as he has done repeatedly this year. Oops, my bad. But, I’m very happy to find intelligent discussion here on this soap opera stock, even though it’s all bad news. And, you’ll have to admit it’s more entertaining than watching the GILD grass grow (note to self – never invest in pharmas which sell actual cures instead of forever maintenance drugs.) Thanks, guys, for such incisive analysis couched in such witty terms.
LikeLike
“Seeking Alpha” is mainly a website for press releases, interspersed with occasional blog-posts. I came across it originally in the context of Ruggero Santilli and his Magnegas companies, where no end of press releases about the radiant future for Magnegas were stovepiped through SA. On the other hand, the same site published a critical blogpost by “Pump Stopper” about company viability, and the amount of shareholders’ money flowing to the Santilli family.
Then Santilli complained, so they withdrew the blogpost, although helpful passers-by republished an archived copy elsewhere.
Anyway, up until January “Joe Springer” was posting regularly at SA with recycled press releases and retirement strategies. Then at the beginning of the year he abandoned other topics and switched to posting how important it is to invest all your money in Cassava. It is almost as if his enthusiasm can be purchased.
https://seekingalpha.com/author/joe-springer#regular_articles
LikeLike
Garbage article , How can you not know about the ongoing open label trial? SAVA just presented this data at the AAIC yet you claim they were performing a secret trial. Why not do your homework first and do a little research before writing your articles. SAVAs open label studies is the biggest thing it has going before P3 trails start 2H21.
LikeLike
Good article. I know nothing about the biology but as a computer guy the case for manipulated images is compelling. To me, nothing would justify the editing of images in a scientific paper unless such edits were called out. But perhaps there are legitimate circumstances that us non-scientists would not intuit? Are there any? What reason might a researcher give to justify such actions.
Secondly, the company claims Simufilam improves the cognitive ability of its patients over time using some industry standard cognition test. I would assume tests such as these would be administered by industry professionals that would not form part of the Cassava inner circle. How easy\hard would it be for these results to also be manipulated.
Thanks.
LikeLike
The open label trial has no control arm. What they found there, is at best placebo effect. But because there is no placebo arm for comparison, you can’t compare.
And the preclinical data is simply fake, by now it doesn’t matter who did it and why.
LikeLike
Oh, but Joe has an article with a chart that demonstrates a rapid decline in the placebo effect for AD patients, so he concluded that SAVA really doesn’t need to conduct a placebo control trial because the wonderful results in the open label have lasted 9 months and it is sure to be the same or better at the upcoming 12 month mark, lol. (I think it will be, too, although perhaps my reason for expecting that might not be the same as Joe’s.) He really had a lot of followers believing earlier this year that SAVA could just skip the unnecessary stage 3 trial and proceed directly to “go” and collect a lot more than $200 under a breakthrough drug designation or the like. Joe has been very effective at levitating the stock price after every tumble, but sadly he may be outmatched this time by the very clever folks responsible for the citizen’s petition. Regarding your disparaging remarks about SA, I think you need to reconsider. SA just provides the forum. The contributors are independent and run the gamut from excellent to awful and from stock pumpers to bashers to neutrals. The commenters are all over the place. I find it very useful. Hmmm. I wonder how Joe would have dealt with your likely comments. And, you could be a contributor of articles yourself. I bet you would have loads of fun.
LikeLike
Thank you for a good summary of recent events.
Not sure if standard practice but can you share a (reducted?) clip of the email exchange with CUNY?
Thanks again and long time coming on Cassava
LikeLike
I’m emailing the same provost to verify that statement. Better not have misled people.
LikeLike
I believe your analysis is as close to the truth as I’ve seen. In no way do I want SAVA to fail. Although I don’t own the stock, I own another AD stock and SAVAs demise is bad for the sector. If it comes out officially that they manipulated the data many investors will shy away from the sector.
Unfortunately, in my opinion, the petition has merit. Wang is in big trouble and SAVA will need to distance themselves as much as possible. There will be tons of volatility going forward and their survival is very much at risk.
LikeLike
I hope you didn’t invest in Athira…
LikeLike
INMB , got many shots on net beyond AD.
LikeLike
Twitter is a special place.
Ages ago, I tried to have this account removed for impersonating me. Twitter eventually decided that it is I who is the fake Leonid Schneider.
LikeLike
Don’t worry, people know, Elizabeth Bik, Leonid, Law firm, all are part of that short selling fake stories syndicate. authorities need to investigate these syndicate.
LikeLike
FDA is accepting Citizen Letters, Glen!
LikeLike
Twitter sucks, anyway. Give SA a whirl.
LikeLike
Why should I generate content for someone else, who like Twitter can later take it away from me and delete it completely?
You all came to this site 😉
LikeLike
An article with thin analysis and loaded with conclusory statements about the use of photoshop and other unverified steps SAVA took to generate and cover falsified data.
All coming from someone who includes in his posting an offer to compensate him for his “journalism” work, via his own website.
Makes me wonder about your motive and generally about any conflict of interests, especially given the content of the article that you just spent 2500 words declaring what would be tantamount to criminal acts at multiple steps in a multi year process. All of which have gone previously unnoticed — except by you and an undisclosed client of a law firm.
If I can borrow a theme from your article, it all seems a little too good to be true.
LikeLike
How do you know my real name is Leonid Schneider?
LikeLike
Please don’t tell me that your real name is “Smut Clyde”.
LikeLiked by 1 person
Great material here. I think we have the makings of a script for a new documentary. I’m thinking along the lines of “The Dumbest Guys in the Lab”.
LikeLike
This is the kind of comments Elisabeth Bik gets. I know i enjoy my male privilege here…
LikeLike
I dont think wealthy people like our presumed boxer Mr Mayweather really understand how high risk these start ups are. I think they assume because its “science” (lol) it has to work. Its more like gambling on a roulette wheel. If its based on fabricated data, well, you’re done.
LikeLike
Hi! You write that the Open label study is not available on clinicaltrials.gov. It is (and has been there for a long time):
“Simufilam (PTI-125), 100 mg, for Mild-to-moderate Alzheimer’s Disease Patients – Full Text View – ClinicalTrials.gov” https://www.clinicaltrials.gov/ct2/show/NCT0438825
LikeLike
You are right, it’s this clinical trial:
https://www.clinicaltrials.gov/ct2/show/NCT04388254
Very weird to describe a trial both “open label” and “Masking: Quadruple (Participant, Care Provider, Investigator, Outcomes Assessor)”.
It’s as contradictory as to describe someone “a very lively dead person”. Open Label means exactly the absence of all masking.
Will correct my text.
LikeLike
Ooops, sorry, forgot the last digit, there.
This is because it starts as an open label study and after 12 months it turns into a placebo controlled “cognition maintenance study” for 6 months, where half of the patients continue with medication and the other half then only gets placebo. While this might be unusual, I think it actually is a legitimate measure.
LikeLike
It’s insane.
LikeLike
For some reason I cannot comment on your comment below. Why do you say it’s insane?
LikeLike
Read the updates to the article above.
There is a reason why nobody else designs trials like that insane open label travesty. No, not because the others are too stupid.
LikeLike
Ok, thanks!
While I have to say that I am not always a fan of your strong, opinionated wording, I do have to say that I appreciate your work here and in general! So, in that sense, thanks, keep it up!
Some minor points / corrections:
That open label study is an extension of this placebo blinded RCT: https://clinicaltrials.gov/ct2/show/NCT04079803
And, as you can see in the changes on the OL study https://clinicaltrials.gov/ct2/show/NCT04388254 they changed the number of participants from 100 to 200 on June 12th, with the last change being made on January 20th. So, some time in between they first announced they would change the n to 150, then to 200, which is their current number. The statement in the press release is a remnant of this.
I do not find this to be damning, unless I’m missing something (at least not nearly as damning as those WBs and the mouse brain).
This is why I’m asking, because I think it’s important to keep allegations realistic in their proportions in order to maintain maximum credibility.
LikeLike
For what it’s worth: Here is the (not accepted) manuscript for that abovementioned RCT with 64 patients, posted on a pre-print server. (At the very least, kudos to them for posting that…)
https://www.researchsquare.com/article/rs-249858/v1
LikeLike
Thanks for that, added to the update!
LikeLike
The press release from 29 July stated: “The open-label study has completed its target enrollment of 150 subjects. ”
While in June, the trial registration was changed to extend recruitment to 200 participants. It would seem a relatively minor issue, but all considered…
That trial is really a bad joke.
LikeLike
Ok, but why exactly is that trial a bad joke?
I mean, I agree on the poster being a bad joke, but the trial itself?
Also, even though that’s a high drop out rate, the drop from 64 to 52 patients in that poster can be explained by a sentence found in it’s method section: “PlasmaP-tau181 was measured in duplicate by SIMOA®, a digital ELISA platform. Data with CVs > 11% were repeated and excluded if > 15% on repeat.”
LikeLike
LikeLike
Gotcha. Yes, they should have done an intention-to-treat analysis!
LikeLike
NAI gave them $2.5 million for the 3-month open label extension. Now that’s demented!
LikeLike
Pingback: Non è il momento – ocasapiens