In early 2020, it used to be a science fact that COVID-19 kills predominantly men, and clever therapies were invented. Female hormones are being proposed to save the men, maybe one day someone suggests chemical castration. More recently, some greedy quacks in Brazil got bored with peddling hydroxychloroquine and ivermectin and teamed up with a US hair loss company, to convince you that the anti-androgen hormone proxalutamide is the cure for the pandemic.
You might laugh, but knowing how Jair Bolsonaro’s regime works, especially regarding quack cures, this bold yet pseudoscientific nonsense is likely to become the national COVID-19 therapy in Brazil soon.
Even these shilling quacks (who always claim to have no financial conflict of interest) are aware that by today, SARS-CoV2 is not seen as a men-killer anymore. It turned out that the (alleged) initial discrepancy of ~70% male ratio COVID-19 deaths was a fluke, for example the current data from Germany (for 2020 and 2021) shows that the difference is not that big, maybe not even that significant. The coronavirus kills both sexes, it even seems its current variants might cause more severe and prolonged disease in young women.
The Feminine Side
Already back in the spring 2020, scientists proposed first cures, with female hormones. New York Times wrote in May:
“Last week, doctors on Long Island in New York started treating Covid-19 patients with estrogen in an effort to increase their immune systems, and next week, physicians in Los Angeles will start treating male patients with another hormone that is predominantly found in women, progesterone, which has anti-inflammatory properties and can potentially prevent harmful overreactions of the immune system.
“There’s a striking difference between the number of men and women in the intensive care unit, and men are clearly doing worse,” said Dr. Sara Ghandehari, a pulmonologist and intensive care physician at Cedars-Sinai in Los Angeles who is the principal investigator for the progesterone study. She said 75 percent of the hospital’s intensive care patients and those on ventilators are men.”
As it is always in science news, when the actual results do not support the headline breakthrough bombshell story, it is not newsworthy anymore. The Cedars-Sinai trial NCT04365127 recruited 40 male patients (22 controls and 18 in progesterone group), those already on ventilation were excluded. On 20 February 2021, the scientists published their results, and neither NYT nor anyone else was interested. This is their recent paper:
Sara Ghandehari, Yuri Matusov, Samuel Pepkowitz, Donald Stein, Tamana Kaderi, Divya Narayanan, Josephine Hwang, Stephanie Chang, Robert Goodman, HeliGhandehari, James Mirocha, Catherine Bresee, Victor Tapson, Michael Lewis Progesterone in Addition to Standard of Care Versus Standard of Care Alone in the Treatment of Men Hospitalized with Moderate to Severe COVID-19: A Randomized, Controlled Pilot Trial (2021) DOI:https://doi.org/10.1016/j.chest.2021.02.024
The authors conclude:
“Progesterone at a dose of 100 mg, twice daily by subcutaneous injection in addition to SOC may represent a safe and effective approach for treatment in hypoxemic men with moderate to severe COVID-19”
This does not really sound like a promised success. And this despite the declaration that “Drs. Ghandehari and Pepkowitz report patent pending on method of use of progesterone agonist for treatment of COVID-19“.
We also learn that the “Study period was shortened to 15 days from the initially 29 days“, that the control group had more patients with obesity and hypertension and that nine progesterone-treated patients were still classified as controls:
“Control patients with significant clinical deterioration (requiring higher supplemental oxygen through high flow devices or mechanical ventilation at any point during the study), or those at Day 7 without clinical improvement were permitted to cross over to receive progesterone therapy. These patients remained in their intention-to-treat group for purpose of analysis.“
In short, progesterone as COVID-19 therapy was a failure.
Another genius approach NYT celebrated in the same article from May 2020 was an oestrogen therapy:
“The genesis of the estrogen trial at the Renaissance School of Medicine at Stony Brook University on Long Island stemmed from a similar observation, said Dr. Sharon Nachman, the trial’s principal investigator, who credited a Stony Brook surgeon, Dr. Antonios Gasparis, with the idea.
The trial enrolled its first patient this past week, and preliminary results could be available in a few months, she said.
“It’s totally out of the box, which is how good ideas often start,” said Dr. Nachman, associate dean for research at the Renaissance School”
This is the clinical trial, NCT04359329, titled “Estrogen Patch for COVID-19 Symptoms” and it’s listed as “recruiting”, almost a year after it started. Dr Sharon Nachman’s institutional website never mentioned any progress on that area of research either. It is likely that the oestrogen therapy, just like the progestogen one, proved useless. Unsurprisingly.
Male Geniuses
Both studies were however run by female investigators, and regular readers of my site know that the biggest and the most successfull COVID-19 bullshitters and quack cure inventors are all male. Didier Raoult and Vovka Zelenko with chloroquine, David Sinclair with “anti-aging” NAD+ supplements, Nadir Arber with cell culture supernatant (EXO-CD24), Jean-Claude Tardiff with colchicine, Michael Holick with Vitamin D, Shai Efrati with hyperbaric oxygen, Jean-Pierre Changeux with nicotine, and almost all others are very virile alpha-males. Their rule is always: take your favourite medicinal product, ideally the one you happen to be selling or shilling for, and declare it to be a COVID-19 game-changer cure. Media loves such stories.
Look, here is another male genius, the French psychiatrist Nicolas Hoertel, who decided that the antidepressant fluvoxamine cures COVID-19, because of the buzzword “cytokine storm”, don’t bother trying to understand why. Hoertel based his verdict on an earlier trial NCT04342663 claiming a kind of success (Lenze et al 2020), which was led by two US psychiatrists, Angela Reiersen and Eric Lenze, to be fair the former is a female bullshitter.
Only men can successfully turn crap into hype. There is no evolutionary psychology in it, just our patriarchal society rewarding male bullshittery and business scammery. And Brazil is definitely a country where patriarchy and toxic masculinity rule supreme.
And look, this all-male team of Brazilian inventors of an anti-androgen therapy succeeded where US women failed. Their approach works, well, in a YouTube video of press conference with a Microsoft PowerPoint at least. This is how they communicated their results last week, as a five-member-strong (pun intended) manel.
A bald scam
Their magic drug is Proxalutamide, an anti-androgen non-steroid hormone patented by the Chinese company Suzhou Kintor Pharmaceuticals, marketed in cooperation by the US company Applied Biology (more on it later), and in urgent need of a medical indication. Before the pandemic, Proxalutamide was proposed by its sellers for the treatment of male hair loss or alopecia, and now, it is declared to be curing COVID-19, Kintor Pharma already received an FDA approval to run a pPhase 3 clinical trial in USA. The pseudoscientific rationale for that will be revealed below. Here is one of the team proudly tweeting the breakthrough:
So there is no paper, no press release even. Hence, some screenshots from that YouTube study:
- double-blind randomized controlled clinical trial with proxalutamide and placebo pills.
- 12 hospitals in Amazonas state
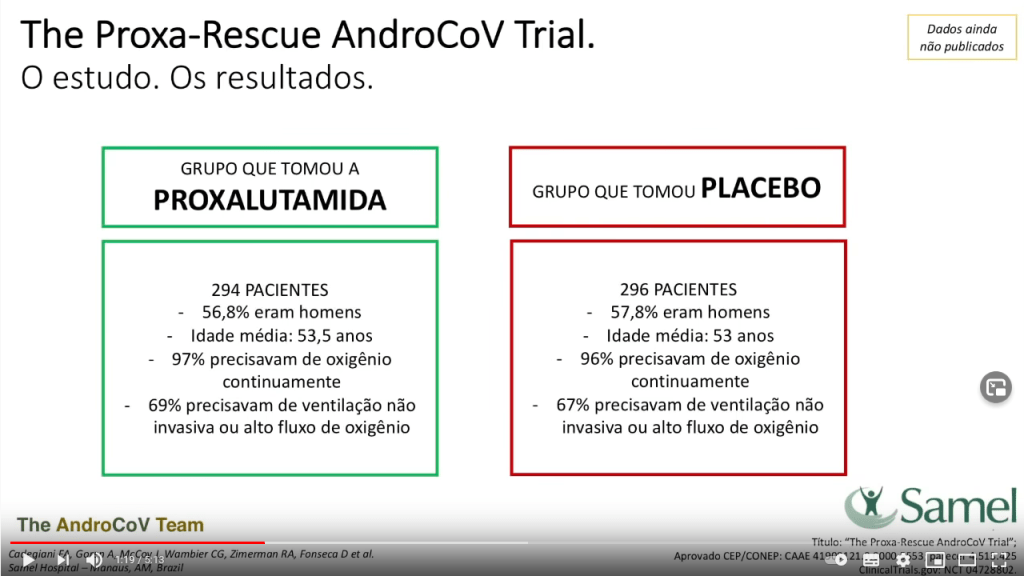
- N= 600 / ITT=590 Proxalutamide N=294 (56.8% male, median age 53,5yo, 97%constant O2, 69% non invasive ventilation); Placebo N=296 (57.8% male, median age 53yo, 96% constant O2, 67% non invasive ventilation.
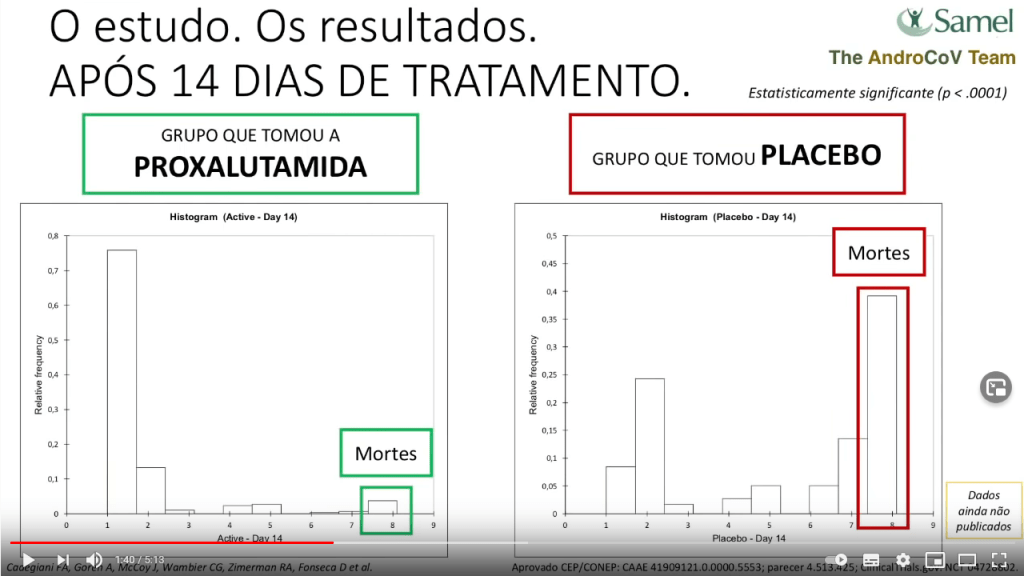
- 3.7% deaths and 80% fully recovered in Proxalutamide arm; 47.6% deaths and <10% fully recovered in Placebo arm. 92,2% mortality reduction (p<.0001)
- Sides Effects. Severe: zero. Most common: increase appetite (25%), diarreia (15%), fatigue (8%), increase libido (7%), irritability (3%), prolonged erection (2%)
- Conclusions: Proxalutamide dramatically reduced the number of deaths and hospitalization time dramatically, inhibited COVID-19 progression, and seems to act on main COVID-19 mechanisms.
The only side effects are increased appetite, both for food and sex, combined with prolonged erection. That in case you, as a virile yet pandemic concerned man, were worried the anti-androgen therapy would affect you masculinity: no, it even increases it while protecting you from COVID-19!
And yet, isn’t it weird that 50% of patients in the control group DIED??? Did the researchers recruit the most severe cases, where hope was already lost? In the video, they do claim that almost 100% patients were on oxygen, 70% of these ventilated. But this cannot be, because the trial registration says something else.
The clinical trial NCT04728802 describes that the patients were admitted if they had a clinical status on the COVID-19 Ordinal Scale of 3 (Hospitalized, not requiring supplemental oxygen – no longer requires ongoing medical care), 4 (Hospitalized, not requiring supplemental oxygen- requiring ongoing medical care) or 5 (Hospitalized, requiring supplemental oxygen)”. Everyone on ventilation, either invasive or non-invasive, was explicitly excluded from the study, and yet now they claim almost 70% were ventilated.
While the YouTube release claims the patients were almost dying, according to trial’s actual records, all treated patients treated had mild to moderate COVID-19. A death rate of almost 50% in the control group makes no sense at all, unless someone is lying. I jokingly asked one of the trialists, Carlos Wambier, a Brazilian-born dermatologist at Brown University in USA, if they recruited Ebola patients by mistake, but received no reply.
Another key character of the proxaglutamide event is Flavio Cadegiani, whose “superlative achievements include amongst the fastest PhD obtained in the history of Federal University of São Paulo (7 months) and concurrent gold medals in Mathematics, Chemistry and Physics Brazilian Olympics in his adolescence.” Basically, Cadegiani is a genius:
“Dr. Cadegiani is the only author of the sole book in Overtraining Syndrome, the prevailing sport-related disease among amateur and professional athletes. He is also responsible for approximately 70% of the articles published in the field in the world in the last 05 years, and reviewer for more than 90% of the manuscripts in the field.”
The quotes are from the website of Applied Biology (getting there in a moment), of which Cadegiani is clinical director. At the YouTube event, Cadegiani said:
“As a researcher, I’ve never seen something like that. […] “I’m one of the editors of one of the Nature journals.“
Surely Nature journals are currently fighting to publish that garbage. No, I am not being ironic. The Seven-Months-PhD-Genius also added:
“Recently FDA has approved our phase 3 Proxa study in the USA and we are going to start Proxa phase 3 in Brazil and ask for ANVISA [Brazilian authorities] for an emergency approval of Proxalutamide. We expect no bureaucracy from them.“
That I definitely believe unconditionally. I can even imagine Bolsonaro and his (soon former) Health Minister, the army general Eduardo Pazuello, already massaging proxalutamide onto their scrotums.
Now meet the most illustrious proxalutamide team member from the YouTUbe event: Ricardo Zimmermann, infectologist at the Hospital da Brigada Militar de Porto Alegre. Zimmerman previously protested in the media against lockdown measures (here and here), while peddling hydroxychloroquine, ivermectin, Vitamin D and zinc as COVID-19 prophylactics. he also made sure that the COVID-19 kit containing those magic drugs became official therapy, distributed to public in the governmental district of Porto Alegre (read here, here) and also watch here:
You cannot be a bigger quack than Zimmerman, and yet look, he now tells everyone to take proxalutamide to survive COVID-19. Now, in the YouTube study, Zimmermann proclaims:
“We are seeing a new chapter in medicine, infectious disease, and anti-androgenic therapy“
No wonder that chloroquine and ivermectin peddlers in US (AAPS) loved this announcement:
Zimmermann is not yet officially on the Applied Biology’s payroll, but who knows. So let’s see who this official sponsor of the proxalutamide clinical trial is.
No conflict of interest
It’s the small US start-up Applied Biology, whose commercial focus before the pandemic was hair loss therapy, hence the anti-androgen hormone proxalutamide this company sells for its Chinese manufacturer Kintor Pharma. It is perfectly clear that the money for the clinical trials comes from China, the Californian company is just a facade. Applied Biology’s official business seat is a mailbox at 17780 Fitch, Suite 192 in Irvine, California, the exact address many other unrelated tiny businesses use. Recall that Cadegiani is employed as Clinical Director there (even though in all of his papers he claims to be solely academically affiliated with Federal University of São Paulo), while Wambier is listed on Applied Biology’s medical board, meaning he is also paid by this company.
If you are still hopeful that this business might be serious and not a scam: one of Applied Biology’s medical board members is the Italian mega-quack and all-kind grifter Torello Lotti, who was even arrested and charged with embezzlement in his home country Italy. Please read for yourself what kind of character Lotti is, here and also here. His speciality is of course hair loss quackery, but no scam is too stupid for him, especially homeopathy, and his grift for gift authorship regularly places him as last author on demented papers about like
- “A Black Hole at the Center of Earth Plays the Role of the Biggest System of Telecommunication for Connecting DNAs, Dark DNAs and Molecules of Water on 4+N- Dimensional Manifold“
- “Recovery of Brain in Chick Embryos by Growing Second Heart and Brain” or
- “5G Technology and induction of coronavirus in skin cells“.

Smut Clyde has prophesied what was coming when he mentioned this paper with Lotti as last author:
“a March-June “Dermatologic Therapy COVID-19” Special Issue at Dermatologic Therapy features Lotti’s name on 21 of the contributions. Notably, Goren et al pursued the idea that testosterone is a risk factor for COVID-19 (as it is for so much else) because mumble mumble angiotensin‐converting enzyme 2 (ACE2) mumble mumble mumble.”
This is the paper, published on 1 April 2020:
Andy Goren, John McCoy, Carlos G. Wambier, Sergio Vano‐Galvan, Jerry Shapiro, Rachita Dhurat, Kenneth Washenik, Torello Lotti What does androgenetic alopecia have to do with COVID‐19? An insight into a potential new therapy (2020) doi: 10.1111/dth.13365
The authors of this genius proposal to treat COVID-19 with anti-androgen hormones “declare no potential conflict of interest”. Andy Goren is Applied Biology’s founder and apparently sole owner, John McCoy is Vice-President, Wambier and Lotti are on the Applied Biology’s medical board, while Jerry Shapiro is another medical board member and dermatologist with exclusive focus on hair loss who runs a private practice in New York and a Hair Transplant Centre in Vancouver, which he himself describes as “One of the busiest, if not the busiest, hair clinic in the world“.
Trials and errors
My heart breaks that Lotti was not included on the follow-up clinical data, a kind of precursor study to what was recently announced on YouTube. This preprint namely:
Flavio A. Cadegiani, John McCoy, Carlos Gustavo Wambier, Sergio Vaño-Galván, Jerry Shapiro, Antonella Tosti, Ricardo A. Zimerman, Andy Goren Proxalutamide Significantly Accelerates Viral Clearance and Reduces Time to Clinical Remission in Patients with Mild to Moderate COVID-19: Results from a Randomized, Double-Blinded, Placebo-Controlled Trial. Cureus (2021) doi: 10.7759/cureus.13492
That clinical study featured 236 patients (108 female, 128 male, with 65 of patients in the control arm) and nobody died there, not even in the usually so deadly control group (remember the recent 50% death rate?) Maybe it was the Brazilian standard of care, which we learn consists of the anti-parasite drug nitazoxanide and Raoult’s antibiotic azithromycin?
But then the data integrity sleuth Nick Brown noticed that there were apparently even more proxalutamide trials going on, done by the same team. Like this clinical trial NCT04446429 where 262 males were treated, with 134 in the treatment group. Only 2 patients in the control group of 128 died there, and unlike in the YouTube study where 50% died in the control arm, even ventilated patients were recruited. What is going on? How many clinical trials did these people run?
Brown found that similar trial data was described by same authors elsewhere, he even noted that “the same percentage of people in both groups were hospitalised in both studies“, referencing this preprint:
Flavio Adsuara Cadegiani, John McCoy, Carlos Gustavo Wambier, Maja Kovacevic, Jerry Shapiro, Rodney Sinclair, Andy Goren Proxalutamide (GT0918) Reduces the Rate of Hospitalization and Death in COVID-19 Male Patients: A Randomized Double-Blinded Placebo-Controlled Trial. Research Square (2020) DOI: 10.21203/rs.3.rs-135303/v1
There, it was not 236 patients, but 214 (100 in placebo and 114 in treatment group), again only 2 in the placebo group died. Oh and of course:
“the Authors declare no conflict of interest with any of the pharmacological interventions proposed by the present study“.
Even although ALL of them, every single co-author on that preprint, owns or works for the Californian hair loss company Applied Biology and directly profits financially from repurposing their drug. Shameless buggers.
Afterwards Brown, with some help from the Brazilian clinical researcher Jose Galucci-Neto, tried to make sense of all these strangely similar yet different trials. He found out that the same ethics approval referred to even more different clinical trials with proxalutamide, with varying and not really compatible numbers of patients, sometimes in combination with another anti-androgen drug, Dutasteride.
The clinical trial NCT04446429 was in July 2020 announced and funded by the Chinese mothership Kintor Pharma, where we learn that
“The experimental arm (50% of the participants) will be treated with Dutasteride + Standard Care (Ivermectin+ azithromycin) and the control arm (50% of the participants) will be treated with Placebo + Standard Care.”
What an interesting drug combination. There is also this:
“Pursuant to the Research Agreement, Applied Biology will study Proxalutamide (GT0918) as an additional arm to the Existing Clinical Trial with around 120 male participants to be enrolled, and is expected to complete the proposed research study in six to nine months of the signing of the Research Agreement.“
The first ever trial participant was enrolled on 20 August 2020, as Kintor Pharma proudly announced. But the numbers still don’t match. By January 2021, final results for the male patients were announced by Kintor, and there were merely “134 male patients in the Proxalutamide arm and 128 male patients in the Control arm“, for women all they had to show were “60 patients in Proxalutamide arm and 35 patients in the Control arm.”
In those control arms, only 2% died. So where did they suddenly get 600 severely ill COVID-19 patients for the YouTube study, where 50% died in the control group? This Kintor Pharma press release from October 2020 admitted that the participants recruited so far were not really ill. And all male. And no way their median age was around 53. But everyone had to be bald:
“For the purpose of exploring the role of anti-androgens in COVID-19 infections, non-hospitalized male participants with mild-to-moderate COVID-19 disease, aged 50 years old or above with androgenetic alopecia, were enrolled in this clinical trial, which is double-blinded and placebo controlled. There are three arms with the first experimental arm treated with Dutasteride + Standard Care (Ivermectin+ azithromycin), the second experimental arm treated with Proxalutamide + Standard Care, and the control arm treated with Placebo + Standard Care. Ivermectin+ azithromycin are used as standard care…”
All of this, if you recall the YouTube presentation and the previous papers, means that someone is lying.
As you see, none of the data has been published in a “peer reviewed” journal yet. I’m sure Torello Lotti will recommend some reliable outlets, unless Nature grabs it first, as Cadegiani hinted.
Oh, and if you follow me on Twitter you might have seen me joking that COVID-19 mortality was associated with baldness, hence my clinical advice to wear a wig. Well, you saw Lotti’s paper with Goren et al. There is more!
Wear a wig
Have you heard of that insane journal-shaped puke-funnel for all possible idiocies, operated by Elsevier and called Medical Hypotheses?
Andy Goren , Flavio Adsuara Cadegiani , Carlos Gustavo Wambier , Sergio Vano-Galvan , Antonella Tosti, Jerry Shapiro, Natasha Atanaskova Mesinkovska, Paulo Müller Ramos, Rodney Sinclair, Omar Lupi, Jana Hercogova, John McCoy Androgenetic alopecia may be associated with weaker COVID-19 T-cell immune response: An insight into a potential COVID-19 vaccine booster Medical Hypotheses (2021) doi: 10.1016/j.mehy.2020.110439
“Our recent work has explored the influence of the androgen receptor (AR) on COVID-19 disease severity [2]. We have elucidated that androgen sensitive phenotypes, e.g., androgenetic alopecia (AGA), are associated with increased disease severity [3]. Here we propose that the dependence of SARS-CoV-2 on the AR may extend to the immune response and might be an important consideration for vaccine development. [..] Taken together, these data suggest that anti-androgen therapy when combined with a vaccine for COVID-19 may improve specific T-cell mediated immune response…”
And again, all these owners and employees of Applied Biology (Rodney Sinclair and Jana Hercogová are medical board members, the latter is top-ranking dermatologist in the Czech Republic) lie without getting red:
“The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.”
The disgusting part is that these greedy scammers use the late Frank Gabrin, the first US physician to die from COVID-19, as their defenceless mascot in order to sell Applied Biology’s anti-androgen hormones as a phony COVID-19 medicine. In the paper Wambier et al 2020 (“Conflicts of interest: None disclosed“) Goren, McCoy, Shapiro and others proposed:
“Dr Frank Gabrin was the first American physician to die of severe acute respiratory syndrome coronavirus (SARS-CoV)-2 infection. Dr Gabrin suffered from androgenetic alopecia and was a long-term survivor of bilateral testicular cancer […] we propose the use of the eponym the “Gabrin sign” to visually identify patients at higher risk for severe symptoms after COVID-19 infection.”

My little joke about hair loss causing COVID-19 deaths became “peer reviewed” insanity and since January 2021 official COVID-19 policy in Brazil:
“The Brazilian government announced on January 6, 2021 that it would adopt the AndroCoV protocol pioneered by Applied Biology in partnership with Brazilian scientists as part of the emergency response to the COVID-19 crisis (https://www.gov.br/saude/pt-br/assuntos/noticias/ministerio-da-saude-prepara-acoes-para-reforco-do-sus-em-manaus).”
It reads as if government announced to deploy a phone app to check if you are bald, so you get treated with proxalutamide as COVID-19 therapy.
And just look at this, as tweeted by Ana Carolina Peçanha:

The article has been updated since it was first published.
Donate!
If you are interested to support my work, you can leave here a small tip of $5. Or several of small tips, just increase the amount as you like (2x=€10; 5x=€25). Those hair loss drugs are not cheap!
€5.00





Lotti and his mates have been pimping dutasteride for a while, trying to find an application for it.
https://onlinelibrary.wiley.com/doi/abs/10.1111/dth.13379
The discovery that it cures COVID-19 was inevitable.
LikeLike
Flavio A. Cadegiani, John McCoy, Carlos Gustavo Wambier, Andy Goren (2021). “Early Antiandrogen Therapy With Dutasteride Reduces Viral Shedding, Inflammatory Responses, and Time-to-Remission in Males With COVID-19: A Randomized, Double-Blind, Placebo-Controlled Interventional Trial (EAT-DUTA AndroCoV Trial – Biochemical)”.
10.7759/cureus.13047
LikeLike
Flavio A. Cadegiani, Carlos Gustavo Wambier, Andy Goren (2021). “Dutasteride Reduces Time to Remission in COVID-19: Results From a Randomized Double Blind Placebo Controlled Interventional Trial (The DUTA AndroCoV-Trial).”
https://www.researchgate.net/publication/345950792_Dutasteride_Reduces_Time_to_Remission_in_COVID-19_Results_From_a_Randomized_Double_Blind_Placebo_Controlled_Interventional_Trial_The_DUTA_AndroCoV-Trial
LikeLike
This one is especially weird:
Flávio Adsuara Cadegiani, John McCoy, Carlos Gustavo Wambier, Andy Goren (2020). “5-Alpha-Reductase Inhibitors Reduce Remission Time of COVID-19: Results From a Randomized Double Blind Placebo Controlled Interventional Trial in 130 SARS-CoV-2 Positive Men”.
https://doi.org/10.1101/2020.11.16.20232512
It’s described as “A double-blinded, randomized, prospective, investigational study of dutasteride for the treatment of COVID-19 (NCT04446429)”. That clinical trial was all about Proxalutamide. This makes no sense.
LikeLike
Another dutasteride result from the same CT of Proxalutamide:
Flávio Adsuara Cadegiani, John McCoy, Carlos Gustavo Wambier , Andy Goren (2020). ”
Dutasteride Reduces Viral Shedding, Inflammatory Responses and Time-to-Remission in COVID-19: Biochemical Findings of a Randomized Double-Blind Placebo Controlled Interventional Trial (DUTA AndroCoV-Trial – Biochemical).”
https://www.researchsquare.com/article/rs-135815/v1
This time only 87 subjects: “placebo group (n=44)… dutasteride (n=43)”.
LikeLike
Oh dear.
LikeLike
I have spent probably too much time today trying to understand what happened with the Dutasteride clinical trial. It evolved in many ways. Notably, between 23 June and 25 July 2020 it acquired a third group of 120 patients, who would receive Proxalutamide (in addition to Ivermectin and Azithromycin). Presumably this was on the insistence of Kintor Corporation, manufacturers of Proxalutamide, who were searching for an application for their drug (in addition to “hair loss” and “androgen-blocker-resistant prostate cancer”), and stepped in to fund the study – as noted in their press release. The job of Advanced Biology was now to find that application.
https://clinicaltrials.gov/ct2/history/NCT04446429?A=2&B=3&C=Side-by-Side#StudyPageTop
Between July 28 and 10 December, the useless Ivermectin treatment was dropped and replaced with Nitazoxanide. I have no idea why.
https://clinicaltrials.gov/ct2/history/NCT04446429?A=4&B=5&C=Side-by-Side#StudyPageTop
Between 10 and 29 December, as the end of the trial approached, the researchers discovered that patients had not been systematically treated with Ivermectin, Nitazoxanide OR Azithromycin, and the ‘background treatment’ aspect was replaced with “Standard of care as determined by the PI”. Patients could be receiving ANYTHING and still be eligible.
Meanwhile, all the outcomes were dropped except “Percentage of subjects hospitalized due to COVID-19”. All other measures? No longer relevant. Suspicious minds might leap to the conclusion that those other measures showed no difference.
‘Presenting “Gabrin sign” i.e., androgenetic alopecia’ was dropped as an inclusion criterion. “Male pattern boldness” was the whole rationale for the study, but it just vanished.
Oh yes, and the Dutasteride group just disappeared. Now only two groups in the study, comparing Proxalutamide with placebo. What happened to all the patients previously treated with dutasteride?
https://clinicaltrials.gov/ct2/history/NCT04446429?A=5&B=6&C=Side-by-Side#StudyPageTop
The result is to make nonsense of various preprints comparing dutasteride and placebo, which refer NCT04446429 for the details and ethics approval. These cite 130 subjects (64 in the D group and 66 placebos), or a subset of 87 who were studied in more detail (43 Ds, 44 placebos).
https://www.medrxiv.org/content/10.1101/2020.11.16.20232512v1
https://www.researchsquare.com/article/rs-135815/v1
But wait, there’s more! A second Applied Biology clinical trial exists for dutasteride: NCT04729491. No ethics approval is mentioned. The study finished in 15 September, and was retrospectively registered on 28 January. Apparently this is possible.
https://clinicaltrials.gov/ct2/show/NCT04729491
No results are presented in the CT entry, but this is the trial cited in other dutasteride papers and preprints. Apparently it is also possible, at the end of a clinical trial, to extract inconvenient cases and reassign them to a second, newly-spawned trial. I don’t make the rules.
LikeLike
Great work!
LikeLike
Hoya Camphorifolia on Cadegiani et al Research Square (2020):
https://pubpeer.com/publications/ABC03819321B564BDDBB15579C092C#1
“The preprint refers readers to Clinical Trial NCT04446429 for further information. The History section of the registration entry for that trial is a fascinating record of changes in inclusion criteria, end-points, and experimental drug : an initial focus on dutasteride morphed into a focus on proxalutamide, possibly due to the arrival of Kintor (that drug’s manufacturer) as a sponsor of the trial, partway through. That need not concern us, though.
What does concern me is that the Results section of the registration entry records 262 total subjects recruited: 134 in the Experimental arm (standard treatment + proxalutamide), and 128 controls (standard treatment + placebo). Of these, 0% and 27% respectively were hospitalised. “Standard treatment” was specified in early versions of the trial, though this later changed into “Standard of care as determined by the PI”, which seems to cover “Anything that the researchers prescribed”.
The preprint, anyway, records that “A total of 214 men were included and completed the trial; 114 men were randomized to the proxalutamide group, and 100 men were randomized to the control group”.
Could the authors resolve this divergence for me?“
LikeLike
Here Cadegiani explains that he doesn’t have any financial COI because a genius like him is above such things.
LikeLike
And here Cadegiani admits there never was a randomized controlled clinical trial, just retrospective pick-n-mix.
Likely of patients who each were given any imaginable drug combination, so they could be recycled for various dutasteride, proxalutamide, ivermectin and azithromycin studies. Where the controls came from, is anyone’s guess. I suspect some unrelated poor souls ventilated at ICUs, for all we know they may have received same drugs.
LikeLike
Look, an actual prescription template by Dr Cadegiani for COVID-19 patients. Without proxalutamide, but with everything else, and more

Must be the “standard care” for his clinical trial’s control arm, you know, the one with 50% mortality.
LikeLike
Isn’t suggesting a financial incentive behind advancing hydroxychloroquine in early treatment of COVID-19 a bit disingenuous, since the HCQ portion of multi-drug therapies costs about $4.76, for all 14 pills needed over 7 days? Dr. Brian Tyson does a good job of explaining his motivation behind his own use of HCQ, in this video regarding his treatment of 1,700 COVID patients, with 0 deaths and only 1 hospitalization of 4 days:
LikeLike
https://pubpeer.com/publications/34FF61C390EA3CC4A5F458CEAFB0B2
LikeLike
A frustrated scientist who makes a living out of attacking others.
He attacks everyone who researches treatments.
It is easy to know the right side. Just watch what Leonid says and be the opposite.
He is always on the wrong side.
LikeLike
Perhaps, but this is what we call an ad hominem argument. If you have a rebuttal to any of Leonid’s points, please share them.
LikeLike
You should know Filipe Rafaeli is a big fan of chloroquine and was last seen calling me a Nazi.
LikeLike
Remember a year ago when Brazil was the poster country for proving that hydroxychloroquine cured COVID and no other treatment would ever be necessary?
LikeLike
Really?? Please, some of us know how to evaluate research…
LikeLike
Pingback: Mi farei vaccinare? - Ocasapiens - Blog - Repubblica.it
This is not to say whether or not the rest of your post is right, but contrary to your statement about higher death rates in males being a “fluke,” it’s a very well established fact that COVID disproportionately kills men. The graph of deaths in Germany by sex that you link to is not adjusting for the population sizes in each age and sex bin – because women live longer and COVID also disproportionately kills older people, this makes a huge difference.
LikeLike
I did some math. From the Staistisches Bundesamt (Federal statistical office of Germany) database https://www.destatis.de/EN/Home/_node.html it can be seen that below 70 yrs the number of women and men is about the same in Germany. For 75 yrs and over, there is about 2 millions more women than men (2.8 mio men vs 4.7 mio women). Now if you look at the COVID-19 deaths by gender and age in 2021 again, the number of deaths is about the same for men and women over 70 yrs (31401 and 30941, respectively). Given that the number of men is about 2.8 mio in that age group and the number of women 4.7 mio, there is indeed a significantly larger proportion of men who die from COVID-19 than women.
LikeLike
Proxalutamide is NOT used to treat hair loss. It is used to treat mCRPC. It is NOT an anti-androgen hormone. It is an androgen receptor antagonist like enzalutamide or apalutamide. Their chemical structures are broadly similar
There is a good peer-reviewed in PNAS, published in Jan 2021, discussing the possible mechanism of enzalutamide and apalutamide in the treatment of COVID-19. https://www.pnas.org/content/118/1/e2021450118 The name Proxalutamide is not mentioned there as the drug is still under a Phase 2 clinical trial for mCRPC in US and 2 Phase 3 trials in China
LikeLike
What kind of Journal is Cureus, then? It has been publishing the most bizarre researches from these authors with no question about data transparency, randomisation methods, allocation concealment and outcome reporting. Not to mention terrible statistics applied on them: authors simply ignored the need of survival analysis – they used t test instead. That is unbelievable! I tried to send a letter to the editor on Cureus, but there is no such option at its website.
LikeLike
Cureus is published according to their website in partnership with MedPage Today.
https://www.medpagetoday.com/
Their editorial board consists almost entirely of men:
https://www.cureus.com/editorial_board
The EiCs are John Adler Jr and Alexander Muacevic, these you should contact via their university emails.
About the journal:
https://www.cureus.com/about
Here the peer review process, which is basically a “DYI” as Cureus calls it:
https://www.cureus.com/author_guide
Meaning, the authors supply their own peer reviews.
If you recall, the proxa authors claim to have no COI while all pocketing cash from selling proxa.
Except that they don’t mean it, apparently.
Why is Cureus, who claim to charge no APC, publishing crap for free? They don’t. Only works from friends and maybe fellow English speakers pass the requirements, the rest:
They don’t reveal the exact costs, because it can be anything.
https://www.cureus.com/cureus_editing_services
It seems once you submitted your article, you cannot withdraw it: “Preliminary acceptance occurs when your article enters peer review”. Basically, you are trapped into buying their editing services. It is a very clever business model.
LikeLike
Well, you guys should check on Cadegiani’s Instagram: he advertised recruitment for one of his trials affirming no need of positive rtPCR, as a new-developed (non-validated) clinical diagnostic tool would suffice. I would not be surprised if many participants on their researches had no confirmed COVID-19.
LikeLike
Leonid, sou brasileira, percebi no seu texto que você insinua que no meu país
estamos vivendo numa espécie de ditadura , cujo ditador
seria Bolsonaro. Nada mais errado!! Ele teve seus poderes limitados para o comando das políticas públicas contra a covid19 por nossa corte constitucional.
A ditadura que vemos aqui e no mundo todo é das big techs, isso sim.
Qual tratamento contra covid19 você propõe ou defende?
LikeLike
Oleandrin.
LikeLike
YOU ARE FAKE NEWS!
https://twitter.com/gilmarmendes/status/1369833851049177088?s=20
LikeLike
Ninguém limitou a atuação do presidente, o STF simplesmente definiu que pela inércia do governo federal os estados e municípios poderiam tomar medidas que achassem adequadas para combater a pandemia.
LikeLike
Senhora, não é questão de defender tratamento. A senhora não entendeu a denúncia contida nesse website? Está ocorrendo uma tentativa de GOLPE da parte desses médicos. Eles inventaram um remédio, deturparam os estudos e agora estão se organizando para vendê-lo ao Brasil. Ao invés de submeter à ANVISA, fizeram um estardalhaço na Internet, para mobilizar a atenção popular. Já tem uma carta da Indústria para o Presidente Bolsonaro desde novembro. Isso é fraude! Querem lucrar em cima da pandemia!!!
LikeLike
Hey, look at this news: https://veja.abril.com.br/blog/radar/aliados-de-bolsonaro-ja-buscam-remedio-substituto-para-a-cloroquina/
So, it seems Dr Cadegiani has already been invited to promote his fake medicine at House of Representatives in Brazil…
LikeLike
Bayesian approach–Pretest probability that any drug, of any kind, represents an active treatment for any kind of virus at all: Low but not zero (see, for example, tx for HIV, Hep B & C, herpes, CMV, varicella). I do note that all of these treatments came about at the tail end of years if not decades of trial and error research. Trial claim: this totally unrelated drug with no rational biologic mechanism is THE BEST for COVID. Post-test probability: Riiiiiight.
LikeLike
Pingback: Le parole per dirlo - Ocasapiens - Blog - Repubblica.it
The number one logical hole in the argument, is that no concrete money flow is proven between Kintor and the California Company.
I would like to see a stronger link proving that they pumped money into the study.
Of course, the death rate stinks to high heaven, undisclosed ties to the California Company, and the mechanism of action does not make any sense that any antiviral will work in the inflammatory phase of covid without being also antinflamatory. Something is way off with the numbers.
LikeLike
To help me understand my logical hole, can you explain how the collaboration of Kintor with AB might work?
You think Goren and Cadegiani pay everything from their own pocket, while Kintor issues press releases to raise investments?
LikeLike
Scenario
Step 1: Open an Interactive Brokers Account under a corporation.
Step 2: Raise cash: Mortgage House(s). Borrow money from relatives. etc.
Step 3: Lever up as much as possible because this is basically insider trading with gray cross-border jurisdictions.
Step 4: Deposit money into IB
Step 5: Buy 9939.HK, Kintor Pharma stock from July 2020 until December 2020.
Step 6: Pump proxalutamide study results to the public, media, internet.
Step 7: Wait to 10x original investment in 6-months from 9939.HK.
Payment for studies: Out of own pocket possibly. Goren and Cadegiani will make 10x in 6-months anyways. Barely any traces. There is also no conflict of interest disclosures for scientists and their stock holdings. Who is going to investigate the brokerage accounts of US and Brazilian doctors/scientists’ holdings of a Chinese pharma company listed in Hong Kong? Under what jurisdiction will this be under? US has the strictest jurisdictions for insider trading in the world, so Goren and the rest of the Applied Bio team has to be careful. Brazil, likely less strict than US. Regulators are usually focused on professionals in the investment/finance industry playing these games at the institutional level. I do not know of convicted insider trading cases involving scientists. If anyone does, please let me know.
Goren’s tax returns will easily show taxes paid on capital gains and so would whatever corporation he traded under. If he was smart he would have used his wife or some distant relative’s name to set up a corporation and stock account.
Brazil tax returns, I am not sure, are foreign capital gains reported?
LikeLike
Pingback: Le parole per dirlo – ocasapiens
As I understand it there are two studies, one with non-hospitalized patients and one with hospitalized. The former is published by Cureus. Is the second one published somewhere so it is possible to see the details?
LikeLike
Hi Karin,
I suggest you read the above comments by Smut Clyde who seem to have figured out what went on there. Nothing trust-inspiring for sure.
LikeLike
I saw that. I actually meant about the results presented as news at different sites, where they cure the patients remarkably fast and efficient.
LikeLike
well, given what we know, we will have to assume that all is just lies…
LikeLike
Well, as a scientist I feel it hard to just assume things… I will be interesting to see where it will be published. If I got that kind of results I would not publish them low-impact with a questionable peer-review process if it wasn’t necessary…
LikeLike
You might recall a Swedish professor at KI, Paolo Macchiarini. Published in The Lancet, several times. Everyone took it as science fact. Did you see my reporting in this regard?
LikeLike
As a Swede I remember this very well. I just meant that most people would aim for a higher impact journal with such remarkable results as this proxalutamide thing. What it means to not even trying but to go for Cureus, I do not know, of course, but it does not increase the credibility, even if the Macchiarini story tells that high impact is no guarantee against fraud.
LikeLike
The problem is that many scientists struggle to believe that some of their peers can be liars, fraudsters, even murderers. The logic being: if it’s in peer-reviewed literature, it cannot be fake. This is why Macchiarini, Wakefield and others were always defended and supported. Including by the editors like Lancet’s Richard Horton who published their peer reviewed fraud in the first place.
In this regard:
https://forbetterscience.com/2020/06/05/would-lancet-and-nejm-retractions-happen-if-not-for-covid-19-and-chloroquine/
https://forbetterscience.com/2019/12/02/the-lancet-unsw-and-khachigians-cancer-cure/
https://forbetterscience.com/2019/03/21/peter-wilmshurst-vs-macchiarini-cult-at-the-lancet/
and this about Göteborg, Lancet is also involved.
https://forbetterscience.com/2018/06/27/gothenburg-to-sack-sumitran-holgersson-requests-7-retractions/
LikeLike
“Bolsonaro’s ‘new chloroquine’ study has evidence of fraud and serious flaws”
https://blogs.oglobo.globo.com/malu-gaspar/post/estudo-da-nova-cloroquina-de-bolsonaro-tem-indicios-de-fraude-e-falhas-graves.html
LikeLike
Pingback: Performing a Covid ‘exorcism’, Chile’s Sinovac woes and anti-lockdown violence in Montreal - Coda Story