The affair around the many data manipulations in papers by US genius of molecular cell biology, the mTOR man David Sabatini of Whitehead Institute at MIT in Boston, was short-lived. No retractions, not much on corrections, and the one or two which were issued always state: “This error does not affect the conclusions of the paper“. Sabatini’s PubPeer record grew since my reporting, but he needn’t worry: presumably the unbiased MIT investigators decided that since Sabatini’s results were financially groundbreaking, Naturally impactful, and most importantly, they surely must have been reproduced by other labs, hence the data irregularities irrelevant. It seems Sabatini was right in calling his PubPeer detractors “steaming turds“.
And because Sabatini’s data is supported by that of his peers, the field of mTOR signalling which he established by co-discovering the gene, once associated with cell growth, now promises to solve all the big diseases, like cancer, cardiovascular issues, obesity, diabetes and of course also old age.
I would like you to meet a couple of very important gentlemen toiling in the mTOR field.
John Blenis
with Stephen Gygi, Lewis Cantley and Andrew Tee
Once in Harvard and now pharmacology professor at Weill Cornell medical school, John Blenis is a very significant researcher of the mTOR field. Would you like to see what he published with Sabatini?
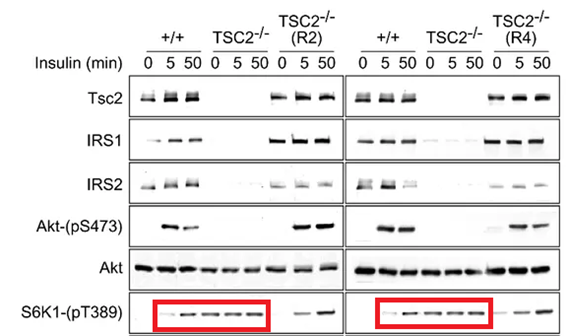
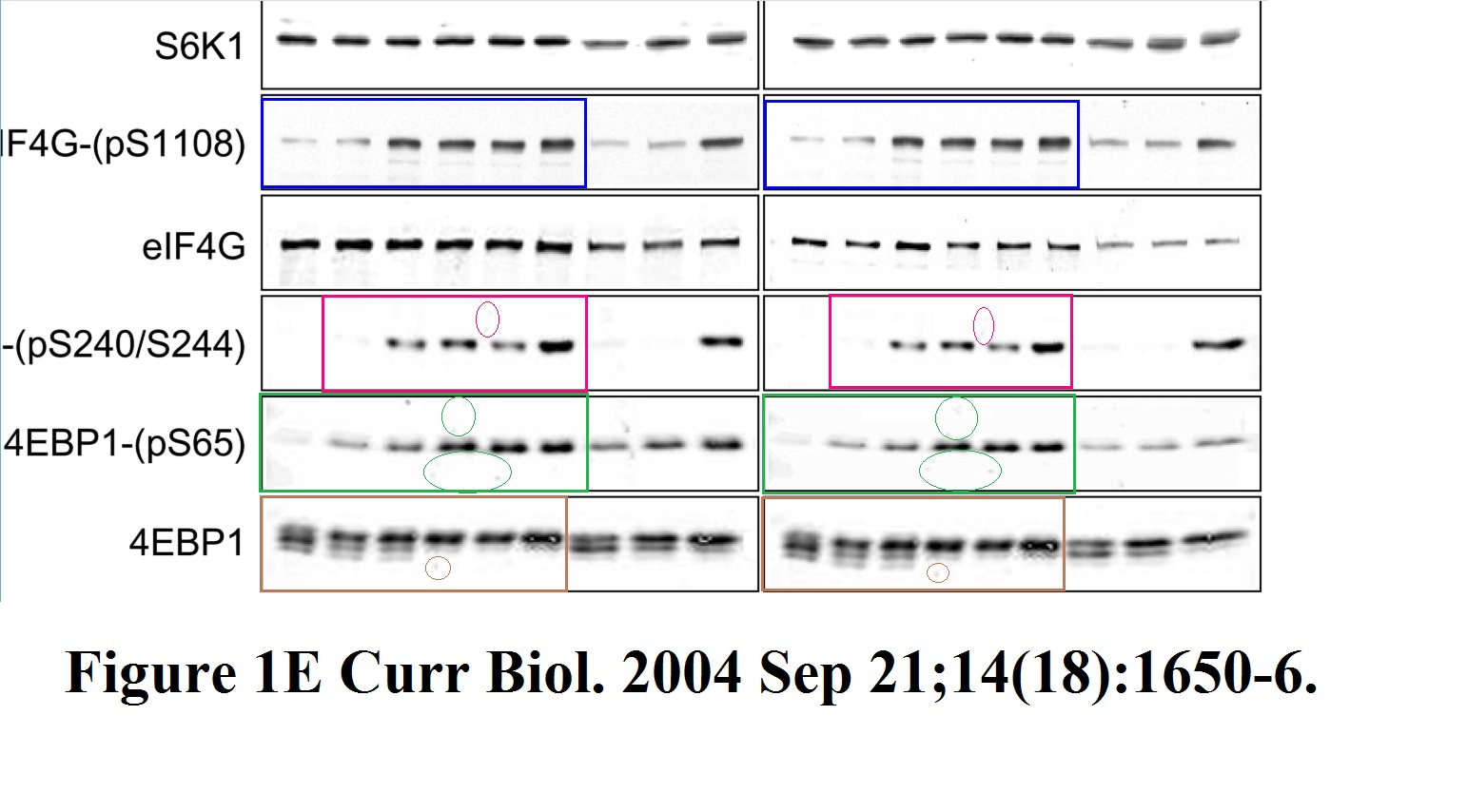
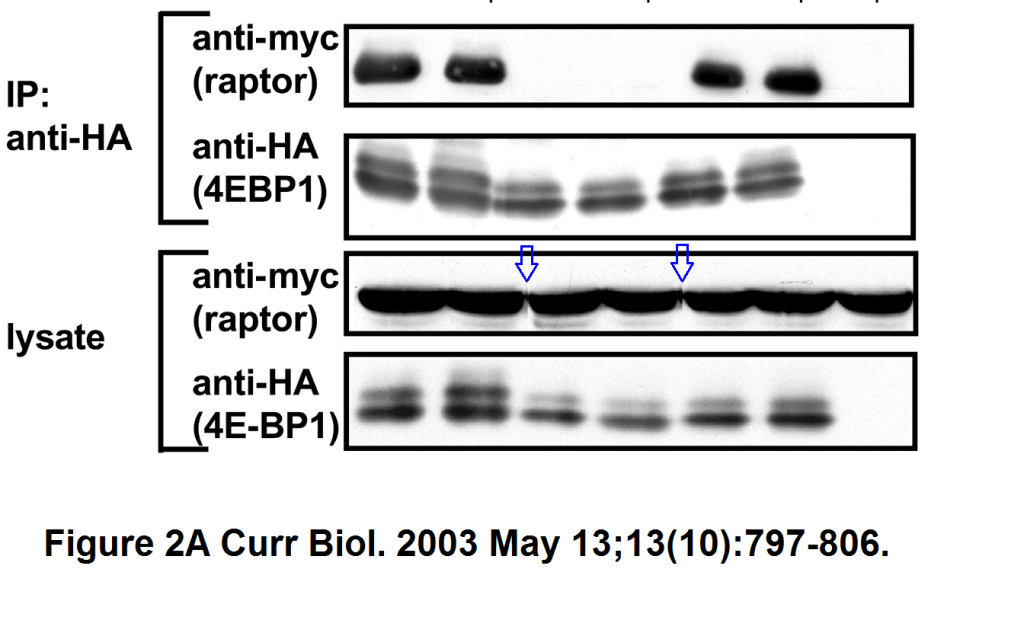
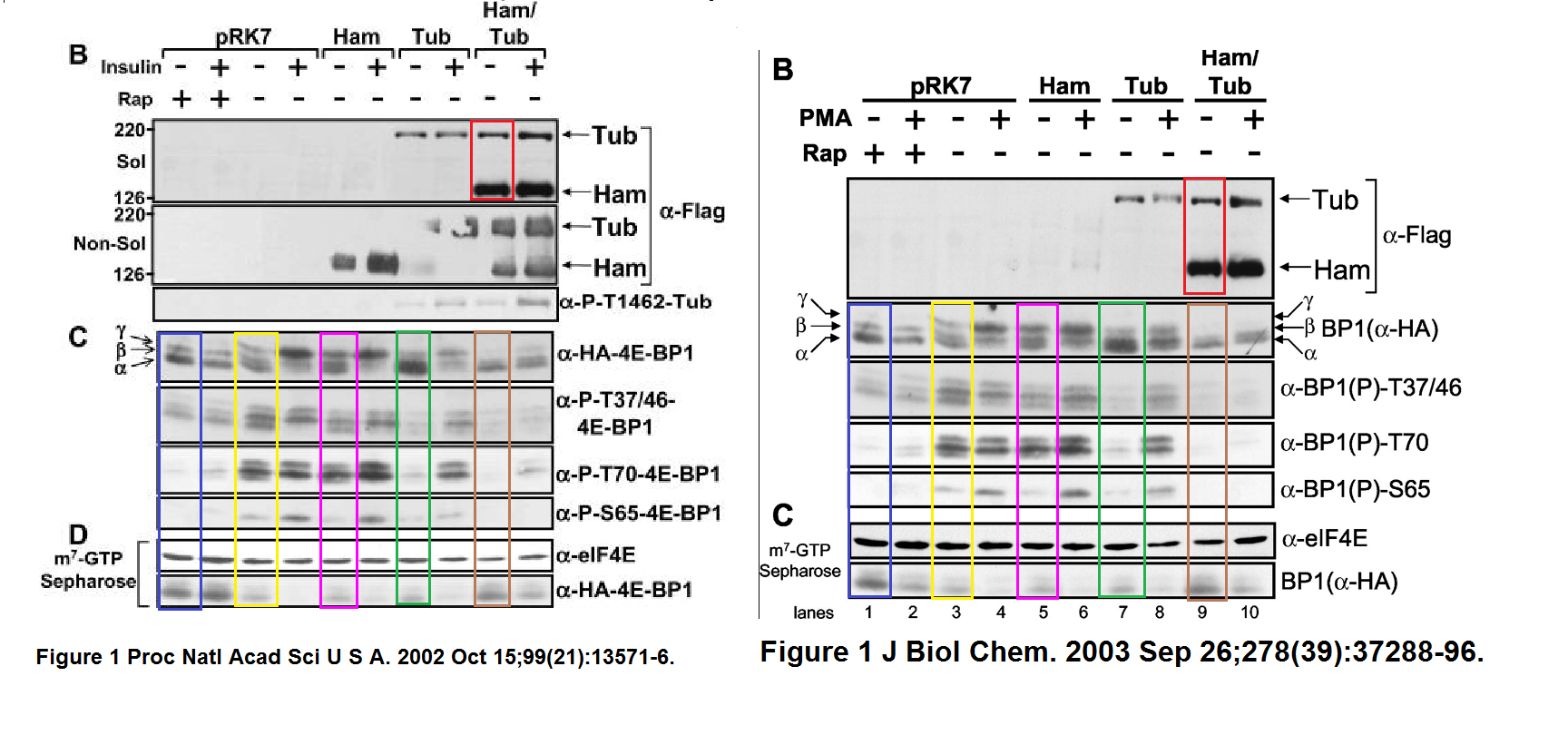
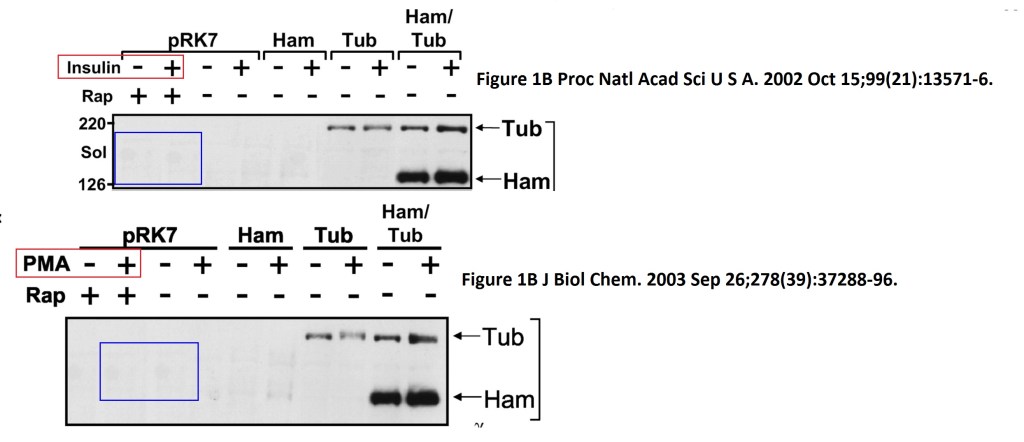
Stefanie S. Schalm, Diane C. Fingar, David M. Sabatini, John Blenis TOS Motif-Mediated Raptor Binding Regulates 4E-BP1 Multisite Phosphorylation and Function Current biology (2003) doi: 10.1016/s0960-9822(03)00329-4
In both Figures two bands were spliced in. What is also worrisome, that the anti-HA panel in Figure 2 D ends with an empty lane stitched on.
It gets better:
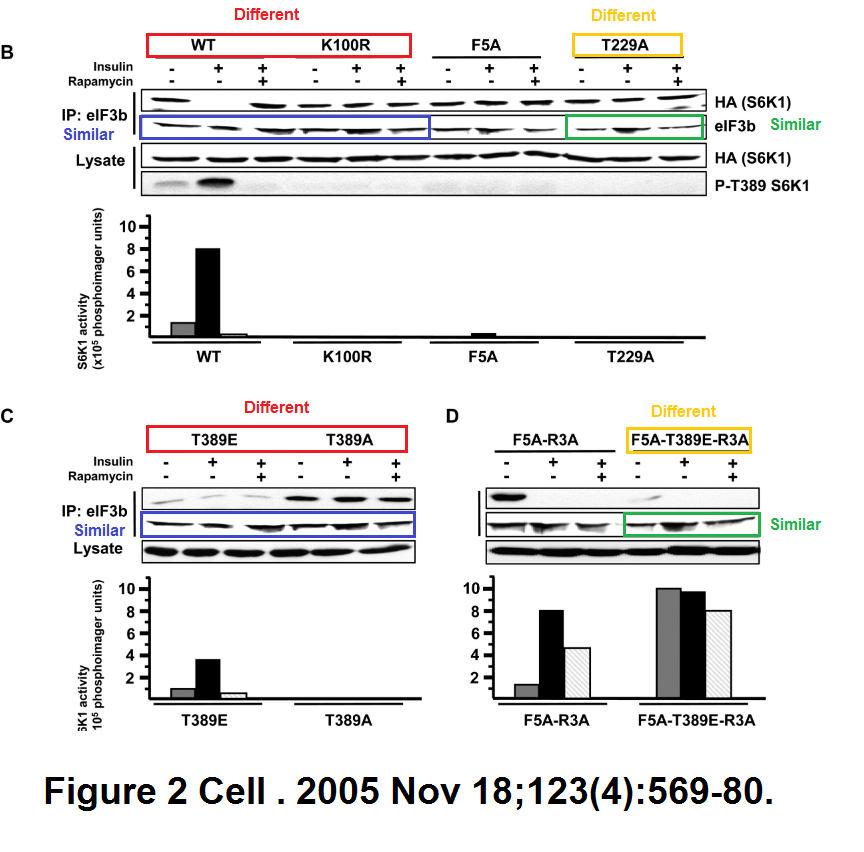
Marina K. Holz, Bryan A. Ballif, Steven P. Gygi, John Blenis mTOR and S6K1 mediate assembly of the translation preinitiation complex through dynamic protein interchange and ordered phosphorylation events Cell (2005) doi: 10.1016/j.cell.2005.10.024
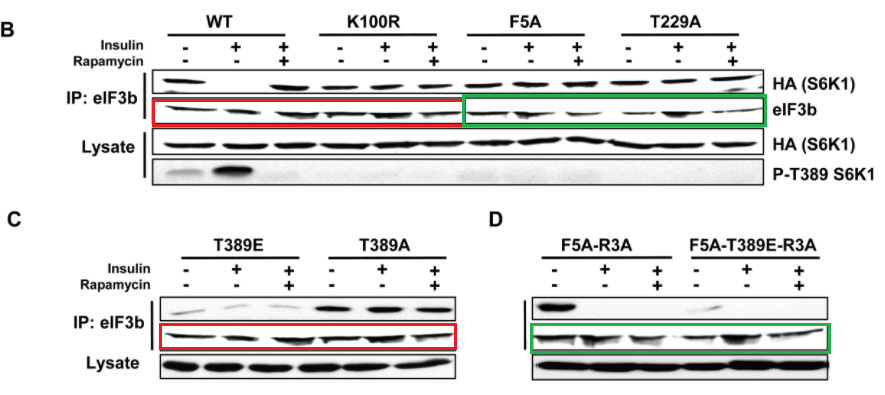
Various takes on Figure 2, with same bands recycled as eIF3b signal for various samples and experiments. But this is in Cell, where such things are actually not just tolerated, but possibly even expected.
Update 16.04.2021: a Cell Press correction was issued for this paper yesterday, in the typical shin-kicking, nose-punching, in-your-face farting attitude of that parasitic Elsevier outlet:
“We, the editors of Cell, were contacted by a concerned reader regarding a duplication in Figure 2 of the above paper. Dr. John Blenis, the corresponding author, determined that the error was introduced during revision of the manuscript. In preparing the final version of Figure 2B, the panel showing results for eIF3b was mislabeled as HA (S6K1) lysate. A different exposure of the eIF3b panels published in Figures 2C and 2D was then mistakenly used for eIF3b, resulting in the duplication.The authors no longer have access to the original data for the HA (S6K1) lysate published 16 years ago, and as a consequence, a Correction is not possible. Given the age of the paper and that the duplication does not compromise the conclusions of the paper, based on the information available to us at this time, we believe that no further action is warranted.”
Steven Gygi is professor of cell biology at Blavatnik Institute in Harvard, his name pops up on several Blenis papers flagged on PubPeer. Gygi however also contributed to problematic papers with Sabatini and Harvard’s titan Bruce Spiegelman, the discoverer of the mythical and likely imaginary slimness hormone, Irisin. Here another study by Gygi and Blenis, also on mTOR signalling:
Sang-Oh Yoon, Sejeong Shin, Yuzhen Liu, Bryan A. Ballif, Michele S. Woo, Steven P. Gygi, John Blenis Ran-binding protein 3 phosphorylation links the Ras and PI3-kinase pathways to nucleocytoplasmic transport Molecular Cell (2008) doi: 10.1016/j.molcel.2007.12.024
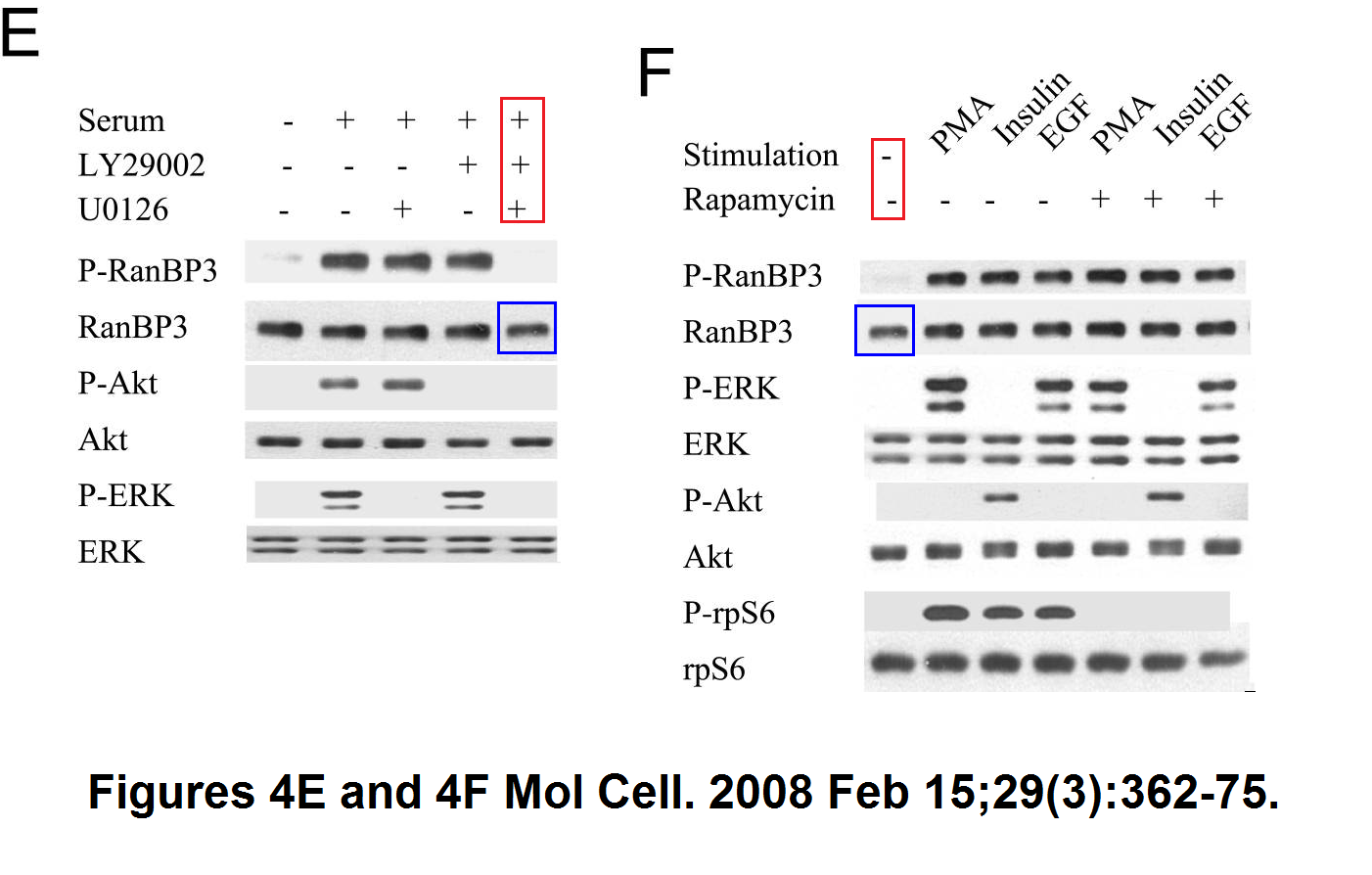
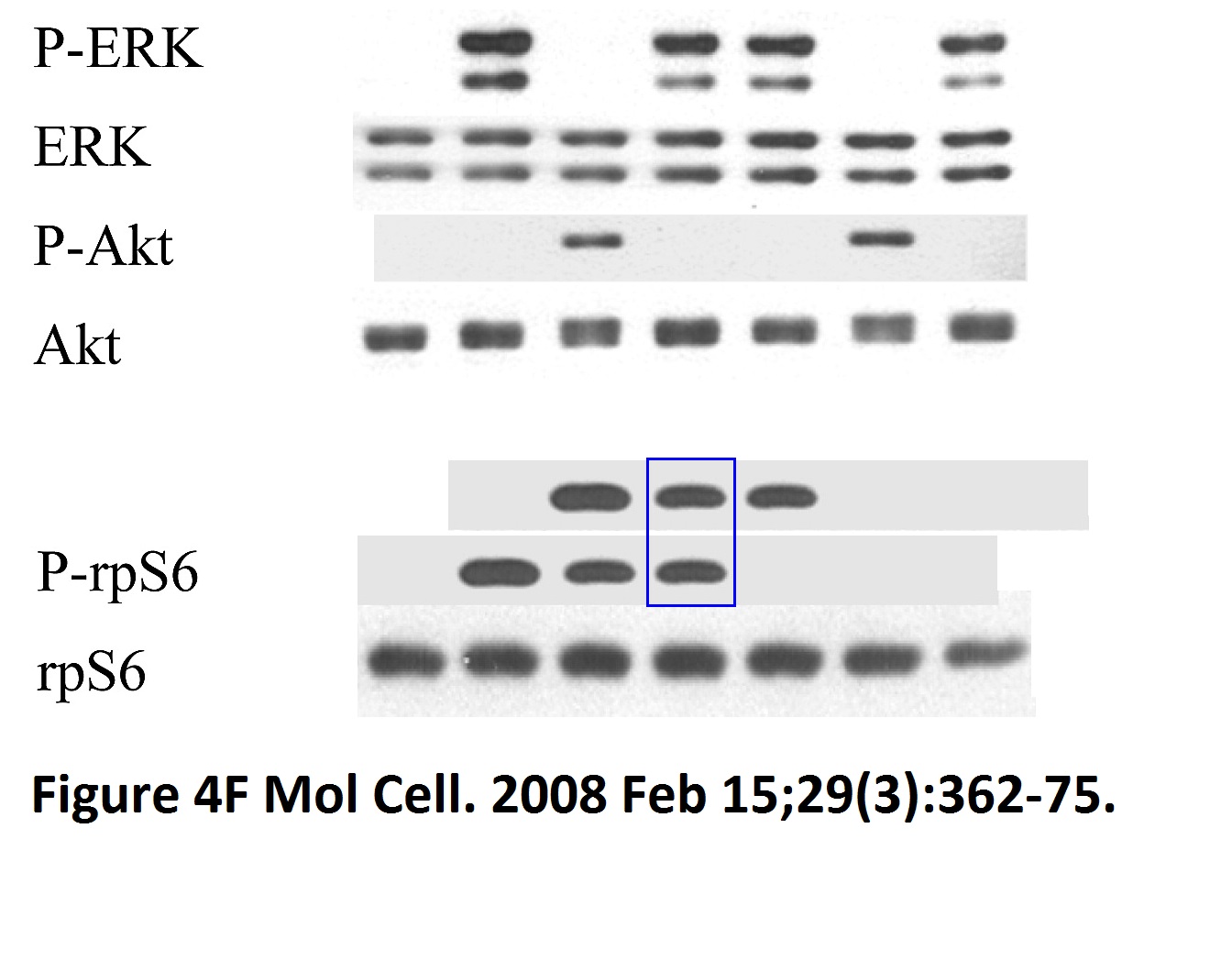
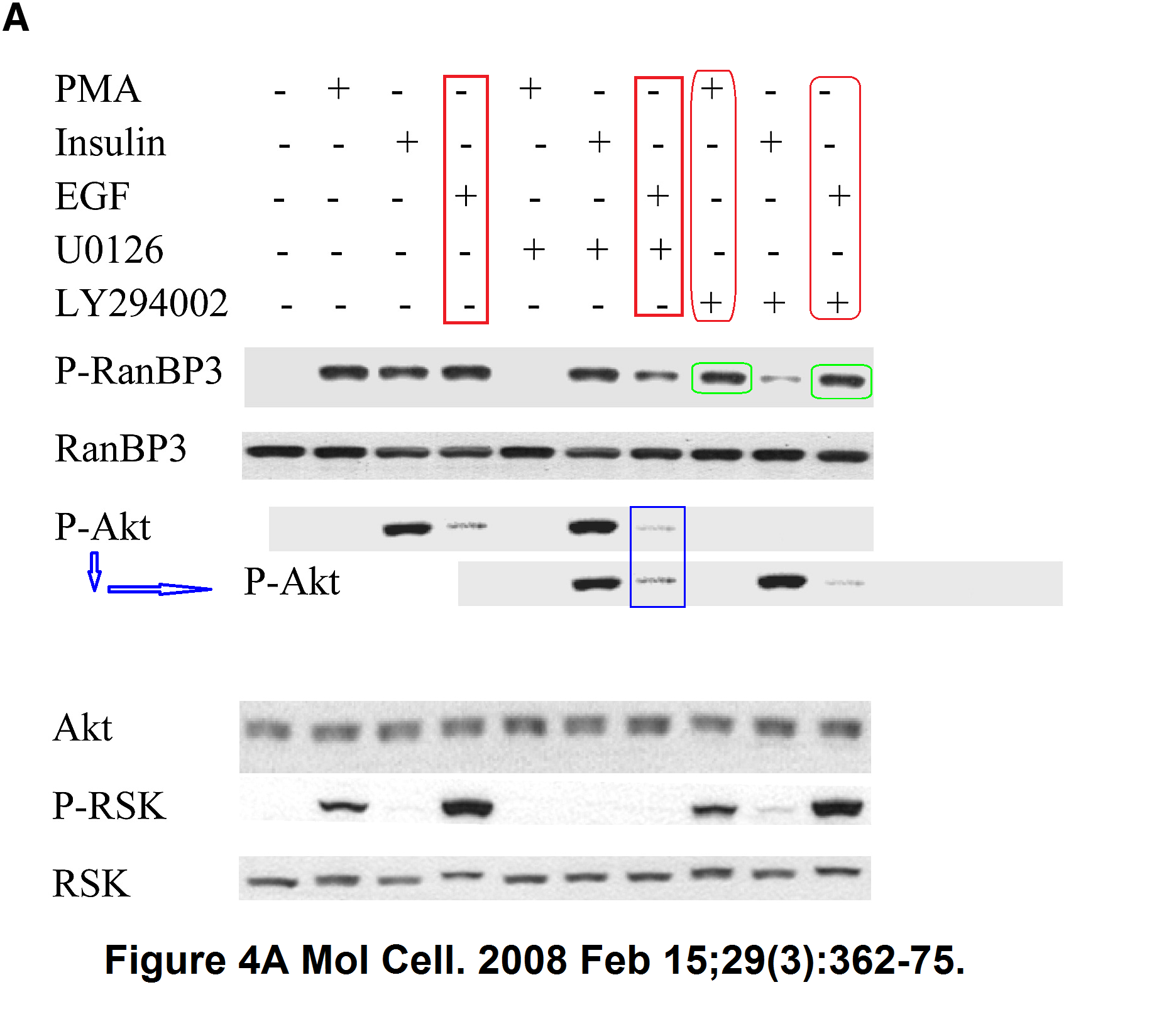
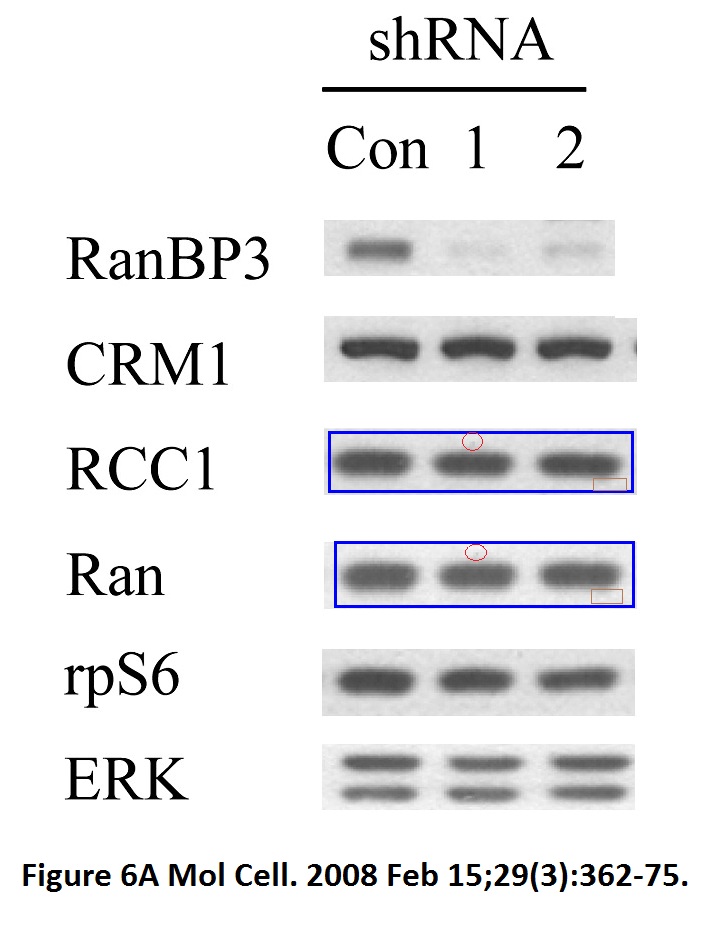
You add some rapamycin to get mTOR going, and look what happens. Bands duplicate themselves willy-nilly. Including in S6K1 kinase, an mTOR downstream effector and a very dodgy protein to make western blot figures with (cf Sabatini lab).
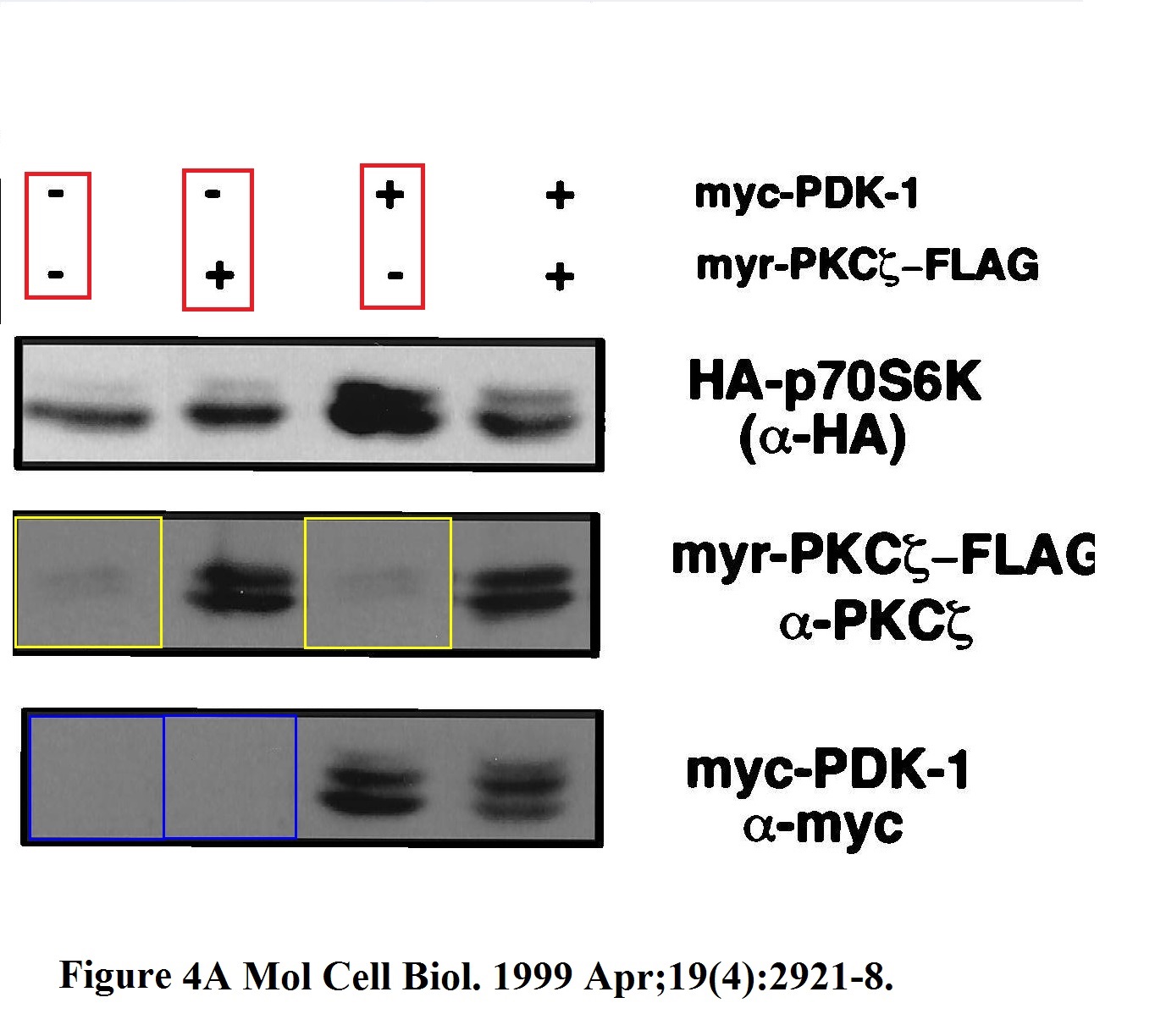
There are some old Blenis classics on Pubpeer, like this 21 year old Romanelli et al Mol Cell Biol 1999 which gels bands look like a stamp collection, with some stamps used twice, probably by mistake of an early edition of the Photoshop. But no time for such vintage classics from Harvard, so here is a rather recent paper from Cornell, so you can see the practice somehow continued.
Jing Li, Alfredo Csibi, Sun Yang, Gregory R. Hoffman, Chenggang Li, Erik Zhang, Jane J. Yu, John Blenis Synthetic lethality of combined glutaminase and Hsp90 inhibition in mTORC1-driven tumor cells Proceedings of the National Academy of Sciences of the United States of America (2015) doi: 10.1073/pnas.1417015112
I find these many cloned bands worrisome, but then again, maybe it is supposed to be like this in mTOR research? Good thing that PNAS is not the kind of journal which would damage scientific progress by retracting papers just because of some fraudulent figures made up in Photoshop. No, Sir. Let’s have some more:
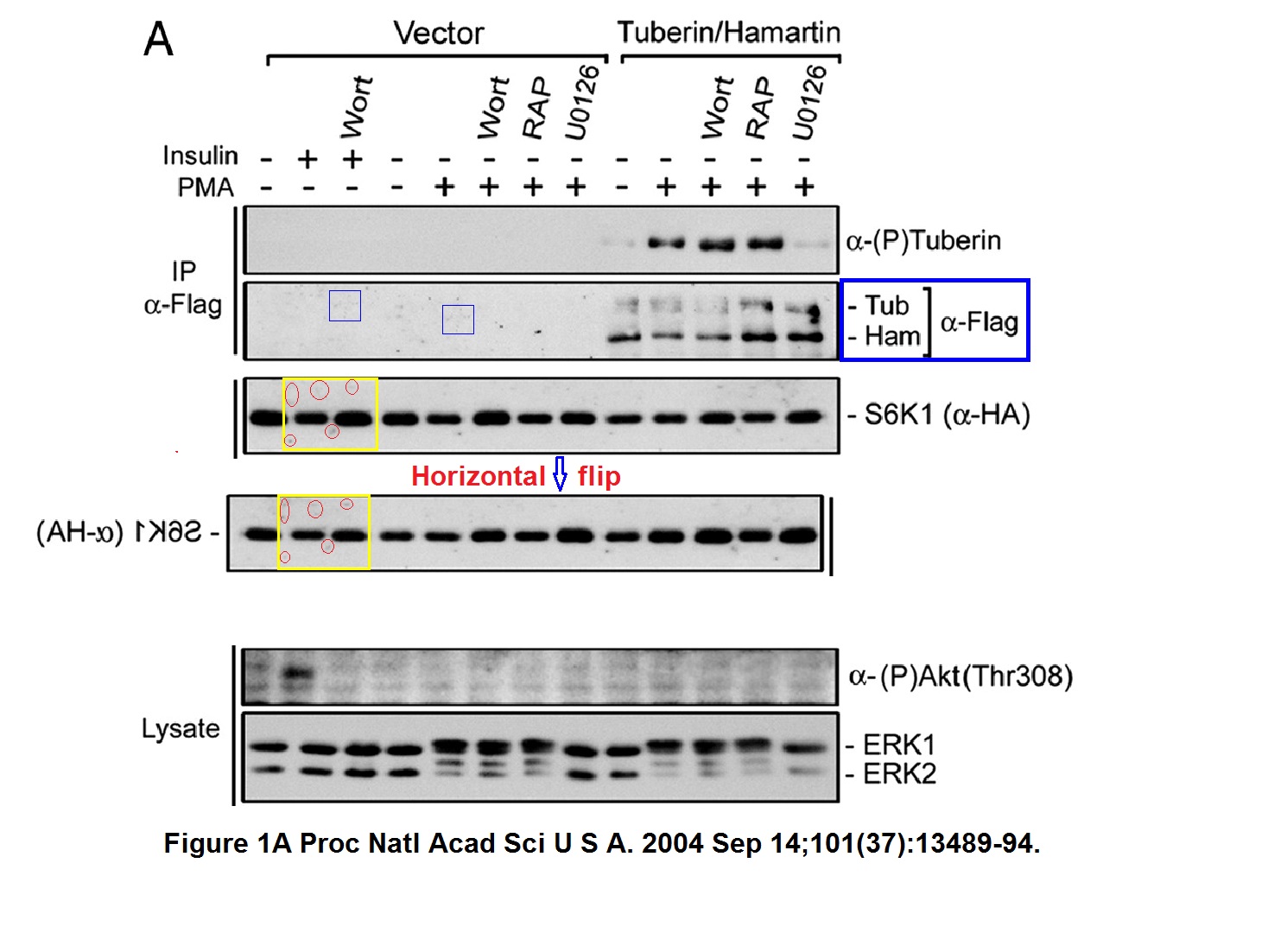
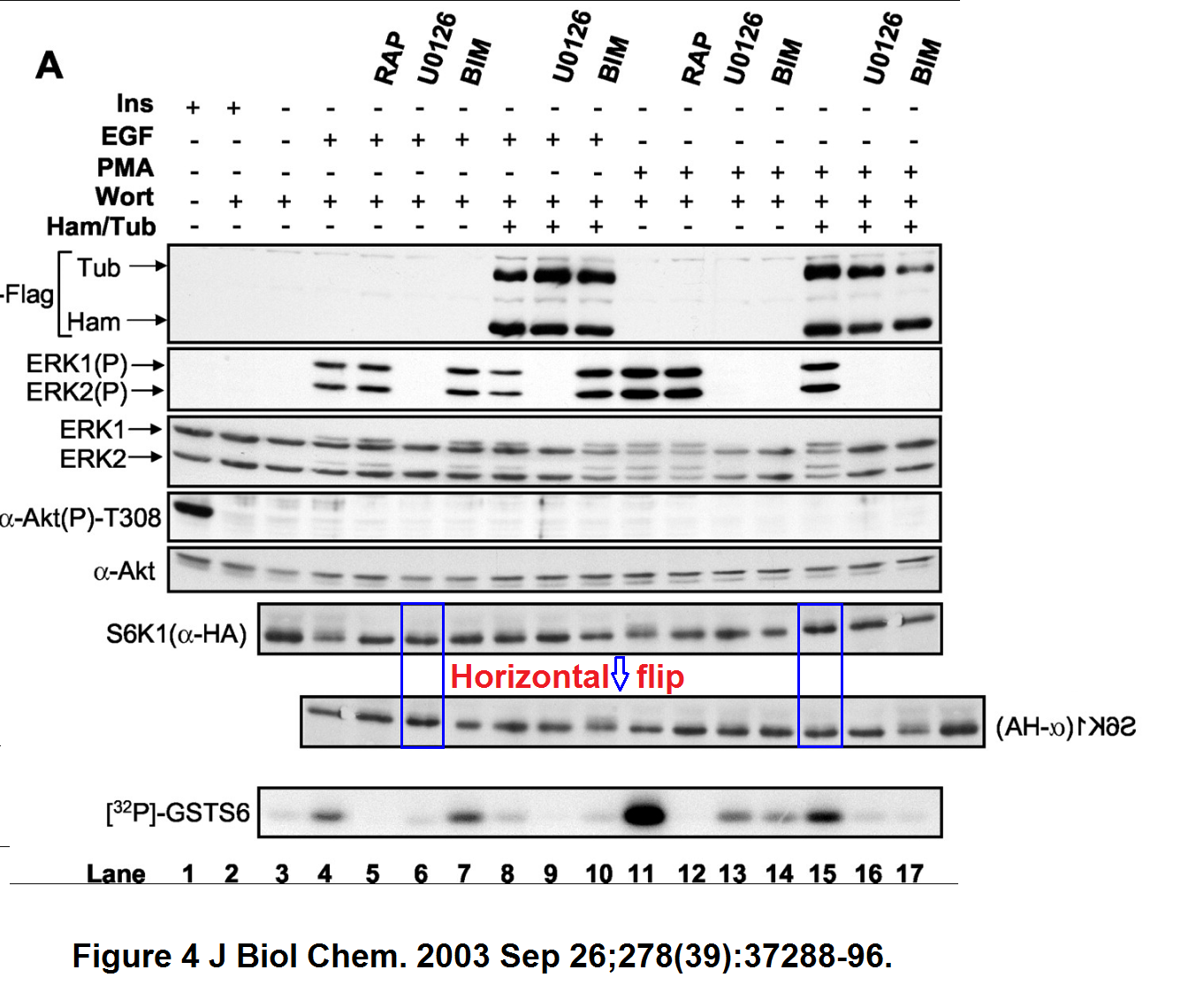
Philippe P Roux, Bryan A Ballif, Rana Anjum, Steven P Gygi, John Blenis Tumor-promoting phorbol esters and activated Ras inactivate the tuberous sclerosis tumor suppressor complex via p90 ribosomal S6 kinase Proceedings of the National Academy of Sciences of the United States of America (2004) doi: 10.1073/pnas.0405659101
A bit of empty background and a pair of bands seem duplicated. Nothing sinister surely, probably there were antibody signals were the researchers did not expect any, and you know how this naughty S6K1 protein behaves. So the bands had to do as told as not to affect the mTOR conclusions. The first author Philippe Roux is now professor at the University of Montreal in Canada, he and Blenis also published this paper:
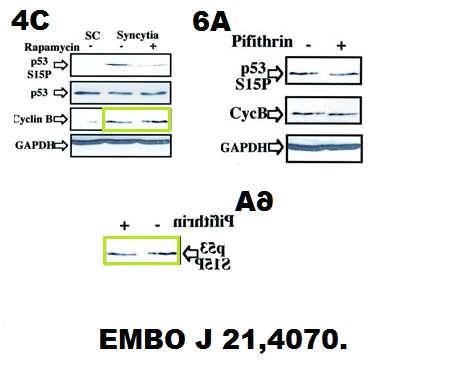
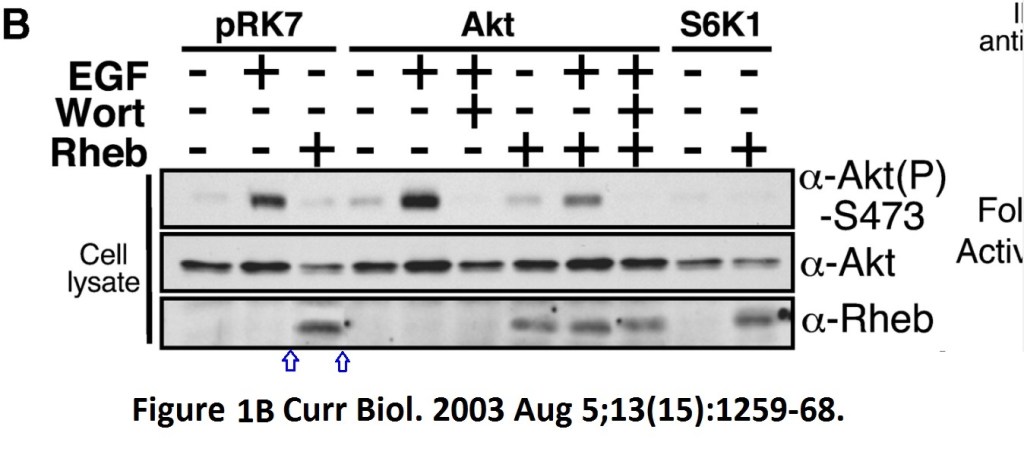
Andrew R Tee, Brendan D Manning, Philippe P Roux, Lewis C Cantley, John Blenis Tuberous Sclerosis Complex Gene Products, Tuberin and Hamartin, Control mTOR Signaling by Acting as a GTPase-Activating Protein Complex toward Rheb Current biology doi: 10.1016/s0960-9822(03)00506-2
Someone very cack-handedly spliced-in a lane in the Rheb panel of Figure 1B. What was wrong with the original signal? And Figure 3 has issues with a duplicated S6K1 lane, but this is OK, since S6K1 protein is known to be dodgy and to cause band duplications without ever affecting any of the conclusions, remember? Actually, are any published S6K1 results real, at all? Better not to go down that rabbit hole.
Now, you might recognise the penultimate name on that paper above: Lewis Cantley, also at Weill Cornell, a real cancer research bigwig who won many awards and prizes, member of various Academies, and is credited with having discovered the PI3-Kinase. Cantley welcomed Blenis’ arrival at Weill Cornell with wine and cheese in 2014. I mentioned Cantley’s own Pubpeer record and presented some of his problematic papers which do not feature Blenis as coauthor in my earlier article about the Wizard Men of Breast Cancer. In fact, Cantley also coauthored some mTOR-relevant papers with Nabeel Bardeesy from Harvard (I once wrote about Bardeesy’s mentee Ruben Plentz), Bardeesy has his own serious PubPeer record. Here a common paper about the discovery of mTOR being regulated by the kinase LKB1:
Reuben J Shaw, Nabeel Bardeesy, Brendan D Manning, Lyle Lopez Monica Kosmatka, Ronald A DePinho, Lewis C Cantley The LKB1 tumor suppressor negatively regulates mTOR signaling Cancer Cell (2004) doi: 10.1016/j.ccr.2004.06.007
Reuben J Shaw, Katja A Lamia, Debbie Vasquez, Seung-Hoi Koo, Nabeel Bardeesy, Ronald A Depinho, Marc Montminy, Lewis C Cantley The kinase LKB1 mediates glucose homeostasis in liver and therapeutic effects of metformin Science (2005) doi: 10.1126/science.1120781
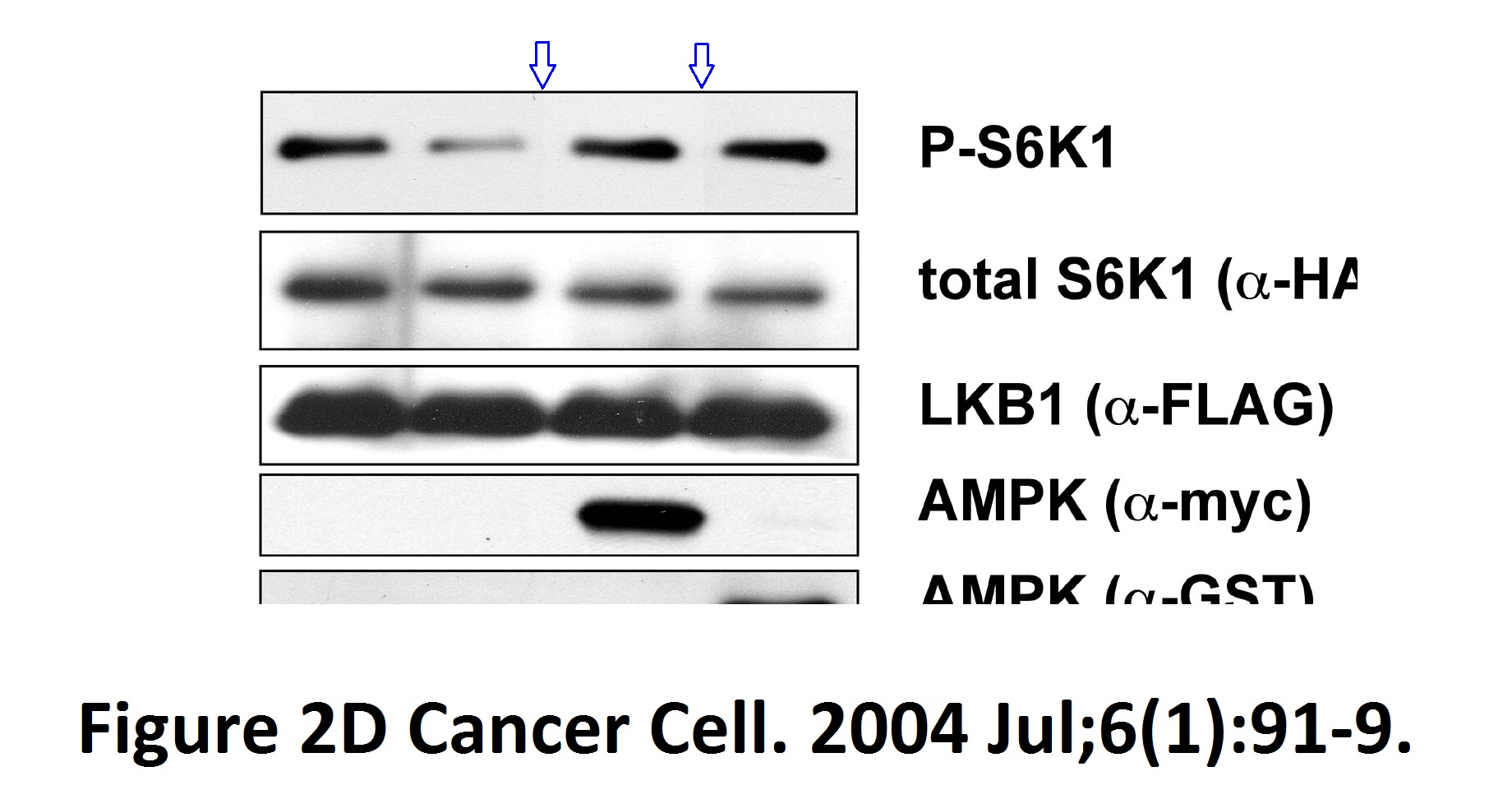
Spliced gels with without proper loading controls and but possibly with duplicated gel bands. Are these top-rank journal papers trustworthy? In this regard, a coauthor on both studies is former MD Anderson Cancer Center president Ron DePinho, with 26 papers on Pubpeer (I briefly wrote about him here). See how it goes, as if there was some kind of mutual attraction between this kind of scientists, you keep recognising the names. In fact, Brendan Manning, coauthor on two Blenis lab papers discussed above, continued with the attitude as a Harvard professor in his own right:
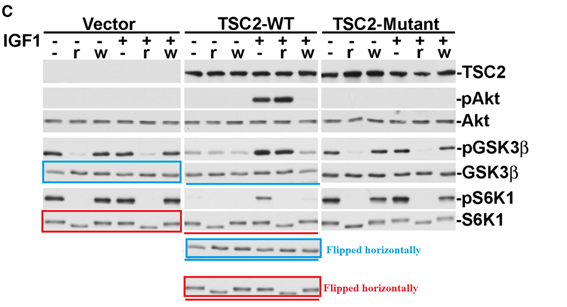
Hui H. Zhang, Alex I. Lipovsky, Christian C. Dibble, Mustafa Sahin, Brendan D. Manning S6K1 regulates GSK3 under conditions of mTOR-dependent feedback inhibition of Akt Molecular cell (2006) doi: 10.1016/j.molcel.2006.09.019
Damn that S6K1, really. The protein can’t be real, no?
But back to the Blenis and Cantley team, which has five mTOR papers flagged on PubPeer. For example, the following study featuring another Blenis’ mentee whom we met above, Andrew Tee, which was personally “contributed” to PNAS by the National Academy of Sciences member Cantley. Even though someone did something very horribly wrong to the figures from a JBC paper which was published by Tee and Blenis just 2 months before:
Andrew R Tee, Diane C Fingar, Brendan D Manning, David J Kwiatkowski, Lewis C Cantley, John Blenis Tuberous sclerosis complex-1 and -2 gene products function together to inhibit mammalian target of rapamycin (mTOR)-mediated downstream signaling Proceedings of the National Academy of Sciences of the United States of America (2002) doi: 10.1073/pnas.202476899

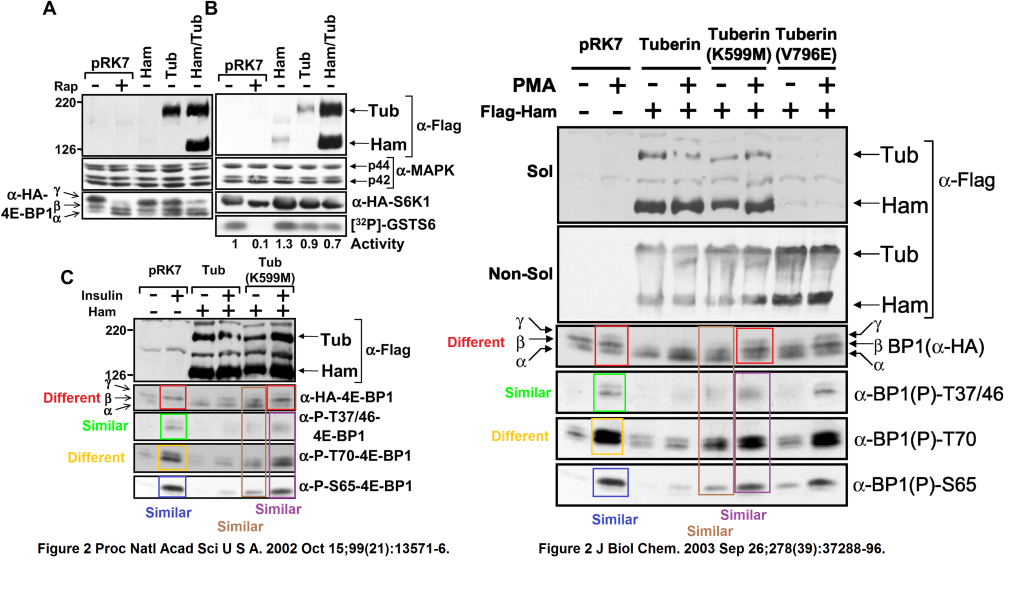
That other JBC paper, ravished, tarred and feathered for the purpose of PNAS submission, was this:
Andrew R. Tee, Rana Anjum, John Blenis Inactivation of the tuberous sclerosis complex-1 and -2 gene products occurs by phosphoinositide 3-kinase/Akt-dependent and -independent phosphorylation of tuberin Journal of Biological Chemistry (2003) doi: 10.1074/jbc.m303257200
It had its own issues of cloned gel lanes, with bands and without:
And now you can guess why Rana Anjum did not make it to the PNAS authors list: the western blot data from her JBC paper with Tee and Blenis “evolved” into something else, when Cantley “contributed” those to PNAS to bypass proper peer review.
Otherwise, the creativity paid off for all involved: Dr Tee is now professor at the Cardiff University in UK, working in mTOR signalling (and perfectly safe there, Cardiff even protects photoshopped TCM quackery).
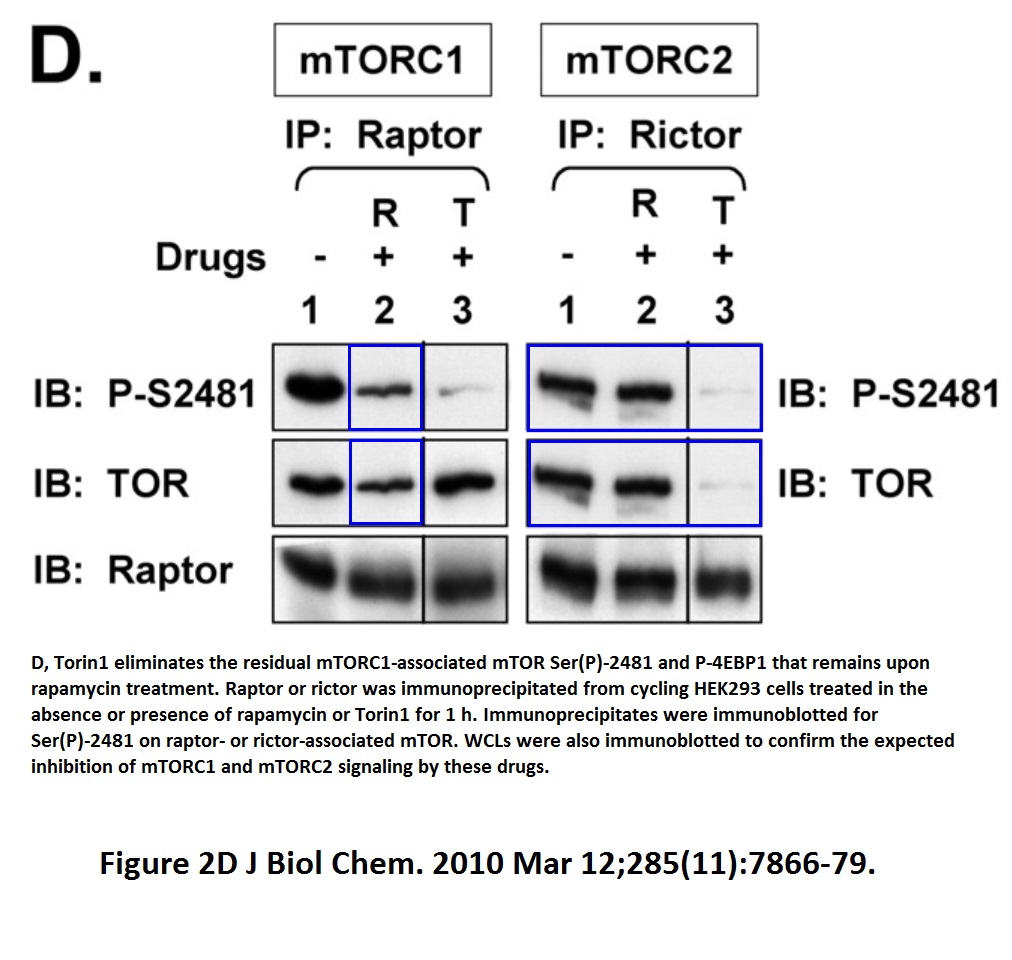
In fact, Tee continued publishing stuff like that, continuing in the mTOR area: look at the Figure 2D from Soliman et al J Biol Chemistry 2010 which he published as newly appointed Cardiff professor. If this is an accident of oversight, then Tee had something in his tea.
But do not expect much action about Blenis’ papers on the side of Weill Cornell either. They not only never bothered about Cantley’s old PubPeer record, they also diligently neglected the unsavoury mess caused by Andrea Cerutti (who incidentally also published some falsified research on the mTOR topic, Sintes et al Nature Comms 2017) and now probably think of the ways to sit out the affair about their own dean, Augustine Choi, who decided that the most toxic gas, carbon monoxide, is actualyl good for your health. You see, once you reach a certain status in academia, the pedestrian rules of research ethics stop applying to you.
A failed scientist is someone who failed to reach this status.
Michael Hall
The Swiss-based mTOR co-discoverer Michael Hall is Sabatini’s counterpart heavyweight in Europe. Even if to a much smaller extent, his lab at the University of Basel, Switzerland, still somehow produced some accidents of oversight in their mTOR research. Seven years ago, Hall corrected a paper in Cell (Thedieck et al 2013), there might be need to correct another one:
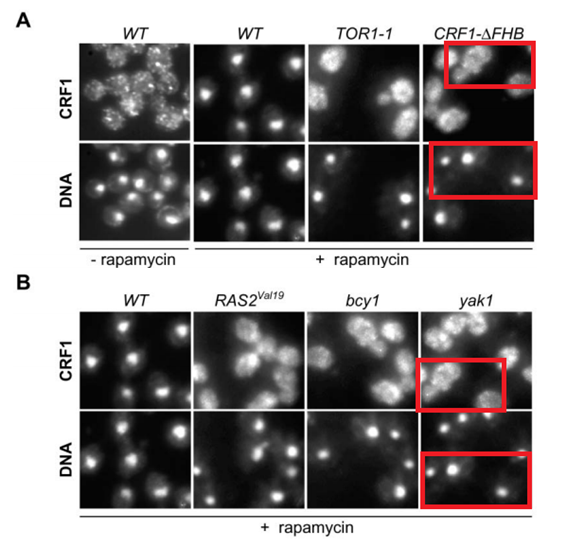
Dietmar E. Martin, Alexandre Soulard, Michael N. Hall TOR regulates ribosomal protein gene expression via PKA and the Forkhead transcription factor FHL1 Cell (2004) doi: 10.1016/j.cell.2004.11.047
How come cells transfected with different genetic constructs start looking so similar?
Also these two papers from Hall lab in Basel might need a correction:
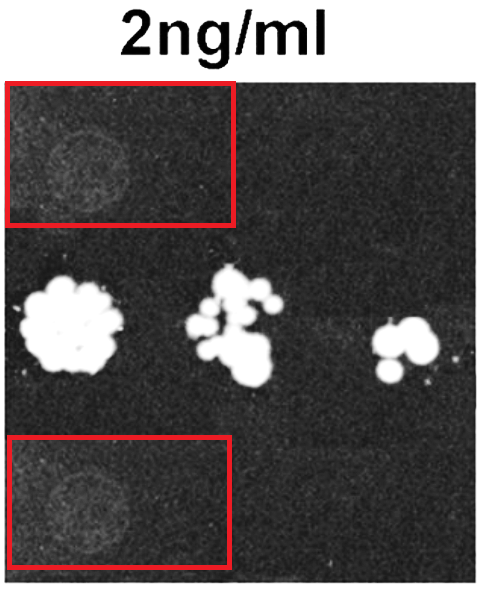

And maybe also this paper could profit from a correction to preserve its conclusions:
Pazit Polak, Nadine Cybulski, Jerome N. Feige, Johan Auwerx, Markus A. Rüegg, Michael N. Hall Adipose-specific knockout of raptor results in lean mice with enhanced mitochondrial respiration Cell Metabolism (2008) doi: 10.1016/j.cmet.2008.09.003
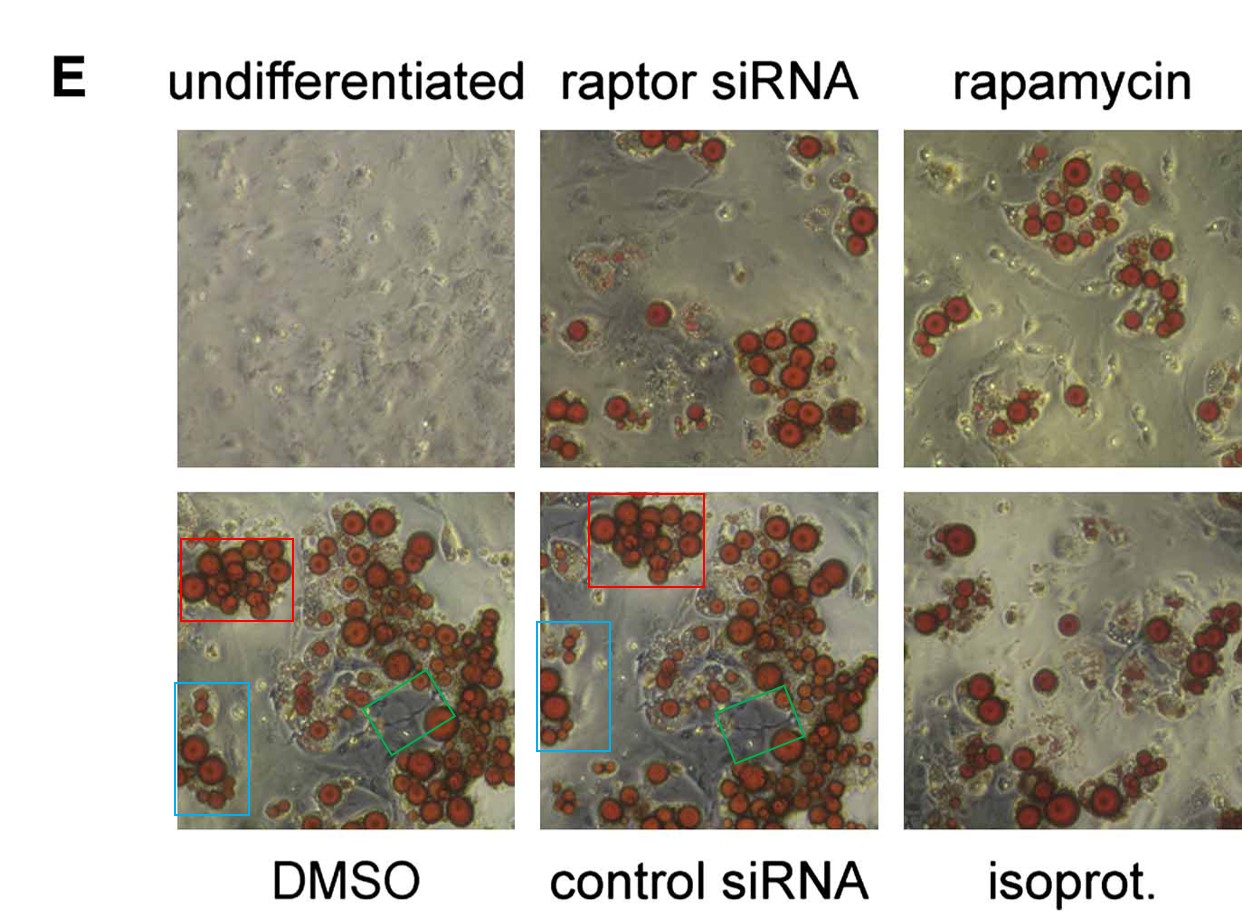
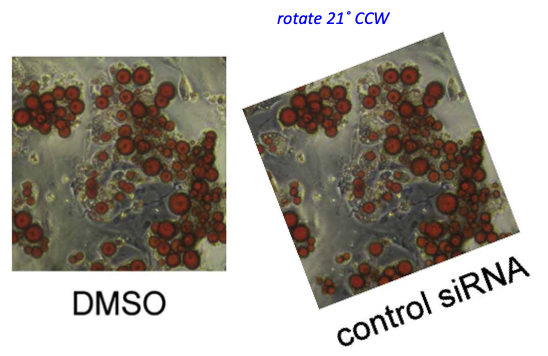
An image, accidentally rotated before reuse. Worth mentioning that the coauthor, Johan Auwerx, is another bigwig in European biomedicine and professor at EPFL in Lausanne, Switzerland, and has 26 papers on PubPeer. In this regard, the only reaction so far was Auwerx’ first author and now EPFL professor Lluis Fajas seemingly taunting his critics on PubPeer. Retractions are something our academic research enterprise reserves for people in Asia, presumably. There were not even corrections for Auwerx (and Fajas). Let’s hope Hall won’t listen to Auwerx’ advice.
Tony Hunter
I wrote about this Gandalf the Wizard of US biomedicine and discoverer of tyrosine phosphorylation before, which earned me a Twitter black by the Salk Institute where Tony Hunter works. So here briefly as small collage from Hunter’s papers on the topic of mTOR and S6K1 signalling, for more read this article.

None of that will ever get a correction, or god beware, a retraction. These works of scholarly learning are safe as houses, just like other problematic papers from Hunter lab at Salk. Some scientists are more equal than others.

Mariusz Wasik
with Erle Robertson
Hence, here another mTOR researcher: Mariusz Wasik, originally from Wroclaw, Poland, now professor and associate director of Fox Chase Cancer Center at Temple University. Yes, same Philadelphia-based university where Domenico Pratico and Antonio Giordano are also professors at, what coincidence.
Wasik has currently 16 papers on Pubpeer, and like with Blenis above, I will sure only the worst mTOR excesses.
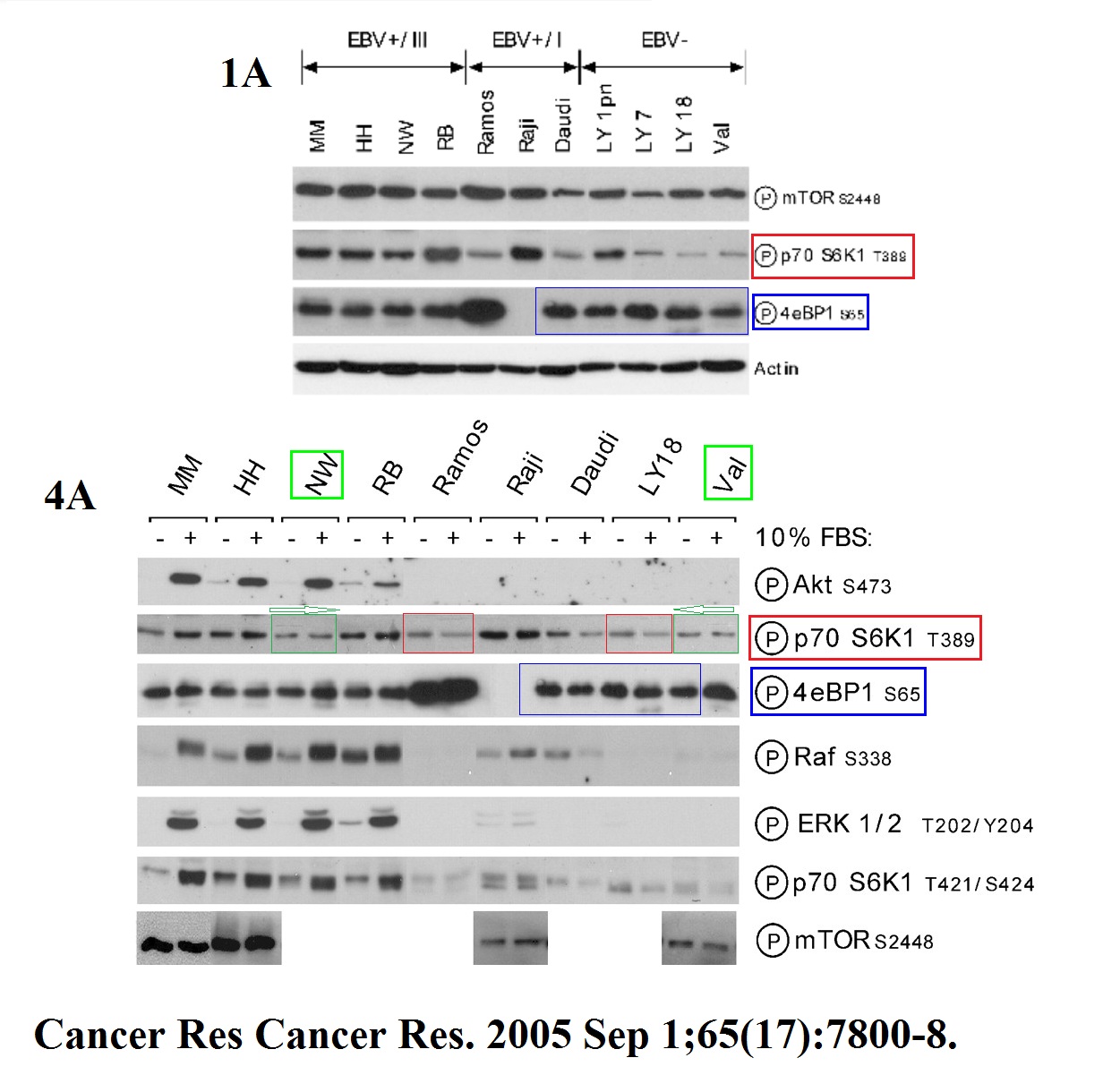
Pawel Wlodarski, Monika Kasprzycka, Xiaobin Liu, Michal Marzec, Erle S. Robertson, Artur Slupianek, Mariusz A. Wasik Activation of mammalian target of rapamycin in transformed B lymphocytes is nutrient dependent but independent of Akt, mitogen-activated protein kinase/extracellular signal-regulated kinase kinase, insulin growth factor-I, and serum Cancer Research (2005) doi: 10.1158/0008-5472.can-04-4180

Kind of reminiscent of the Tee & Blenis artworks, no?
A coauthor of this paper is Wasik’s Philly neighbour Erle Robertson, virology professor at the University of Pennsylvania. This gentleman has his own Pubpeer record of almost 50 papers. There is a massive evidence of hair-raising data fakery in the papers from Robertson lab, so you will be surely reassured that he is now fighting on the COVID-19 front and “has developed a “PathoChIP”, which is a metagenomics assay for parallel DNA and RNA detection of all known viruses, and a comprehensive group of human pathogens which include bacteria, fungi, protozoa, helminths.“, as the Medical School at UPenn proudly reported. I would not trust that assay much, it probably runs on Photoshop.
So here another Wasik-Robertson copro-duction on the topic of mTOR signalling:

Mouna El-Salem, Puthiyaveettil N. Raghunath, Michal Marzec, Xiaobin Liu, Monika Kasprzycka, Erle Robertson, Mariusz A. Wasik Activation of mTORC1 signaling pathway in AIDS-related lymphomas American Journal Of Pathology (2009) doi: 10.2353/ajpath.2009.080451
More? The two men are really a dream team:
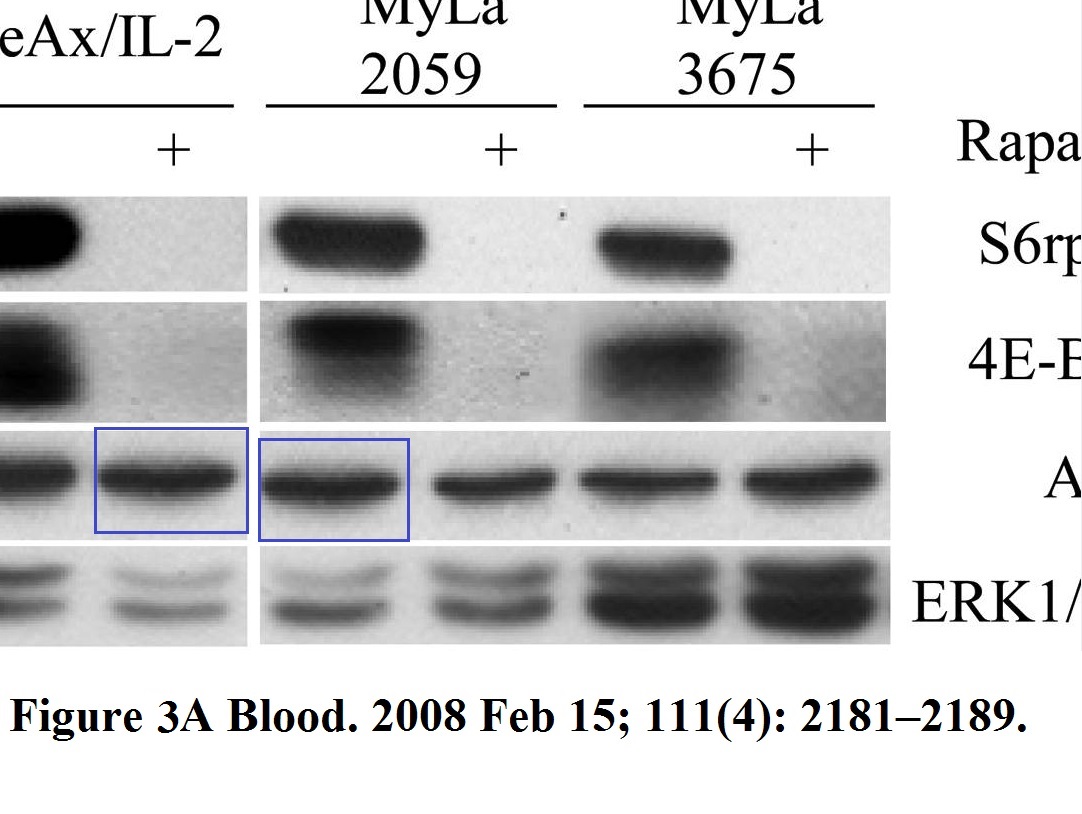
Michal Marzec, Xiaobin Liu, Monika Kasprzycka, Agnieszka Witkiewicz, Puthiyaveettil N. Raghunath, Mouna El-Salem, Erle Robertson, Niels Odum, Mariusz A. Wasik IL-2– and IL-15–induced activation of the rapamycin-sensitive mTORC1 pathway in malignant CD4+ T lymphocytes Blood (2008) doi: 10.1182/blood-2007-06-095182


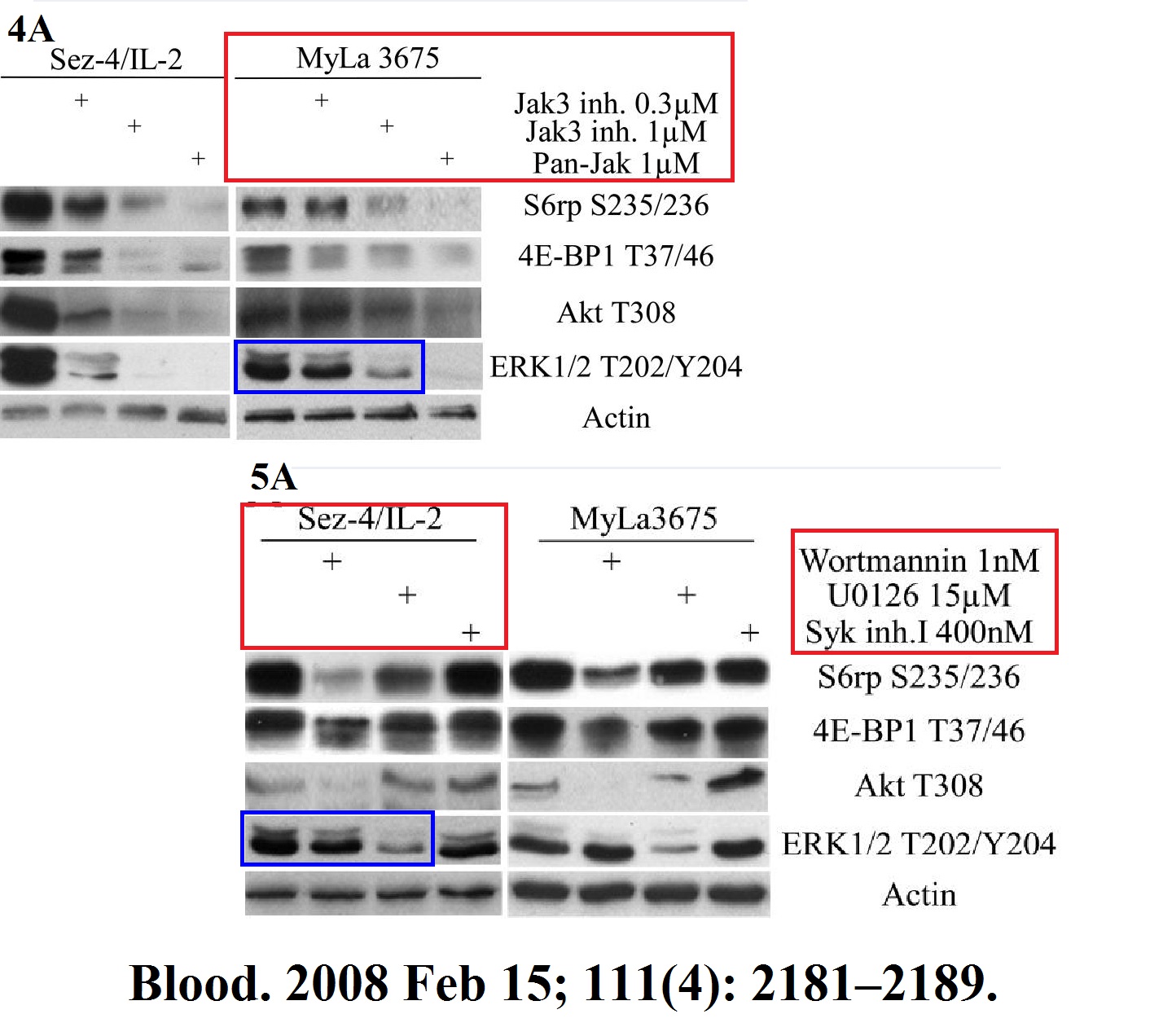
It’s not all about mTOR of course, look here is HIF1 field, for which Gregg Semenza was awarded with the Nobel Prize. Wasik’s research there follows Semenza’s pattern:
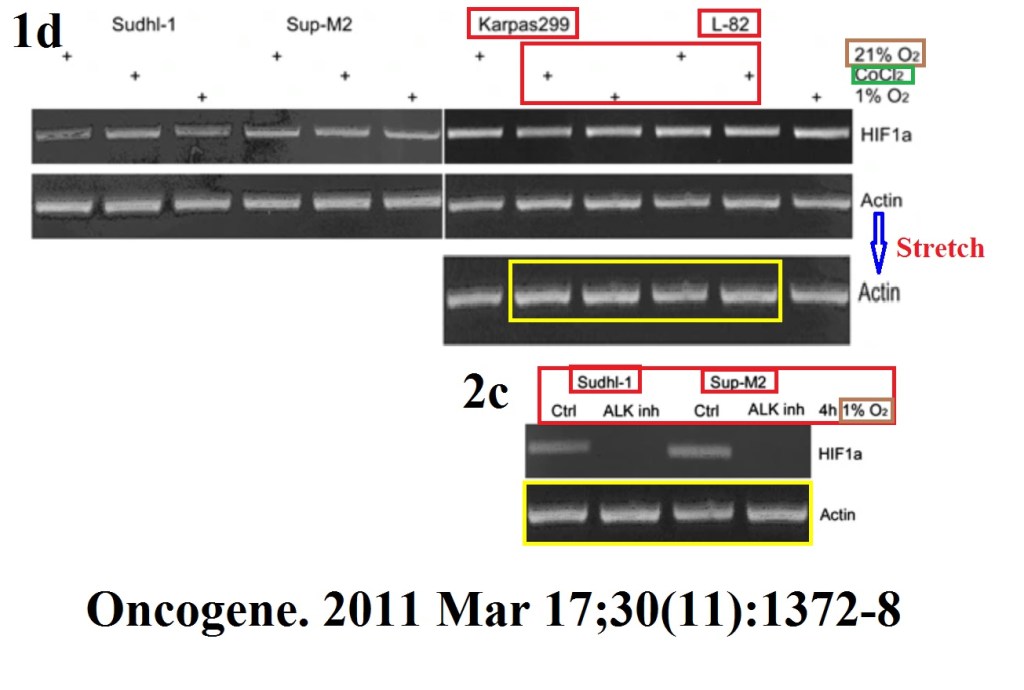
M Marzec, X Liu, W Wong, Y Yang, T Pasha, K Kantekure, P Zhang, A Woetmann, M Cheng, N Odum, M A Wasik Oncogenic kinase NPM/ALK induces expression of HIF1α mRNA Oncogene (2011) doi: 10.1038/onc.2010.505
A paper from Wasik’s lab, featuring same first author Michal Marzec (now associate professor at University of Copenhagen, Denmark) Marzec et al PLOS One 2011 was recently retracted, for data manipulation. Professor Wasik went to PubPeer to protest:
“We find the retraction decision unjustified, based on the available data. While we do not have the original films due to the passage of time (> 9 years since the publication) […]
- the questioned 4 bands are negative controls without a significant impact on the study’s results and conclusions
- this publication has gained 57 citations, all confirming and extending conclusions of the study with none questioning its results
[…] In summary, while we appreciate and have always taken very seriously the need for research integrity, we find the editorial decision in this case to be very harsh and unjust…”
Indeed, as Sabatini said:
It’s just another hot field
I put “title:mTOR” into Pubpeer search function and came up with 235 results, and that is just for paper titles. Much of it from China, which may just as well be utterly fictional fabrications by paper mills. But some names ring a bell. Luca Maria Neri and Giorgio Zauli, for example, from the University of Ferrara in Italy, people not to be messed with.
There will never be a single retraction for Neri and his boss and coauthor, the University of Ferrara rector Giorgio Zauli, despite a jaw-dropping PubPeer record of data fakery. Not just because Zauli is friends with Italian fascists (yes, literal fascists), but because he sues everyone. The Italian journalist Sylvie Coyaud was reported by Zauli to state prosecutor and fined for… my own writings on my site and Twitter. She only learned of all that when the fine was delivered.
Among other names popping up in the PubPeer search is another Italian genius, Pier Paolo Pandolfi, who lost his job in Harvard while trying to “win” a female postdoc (read here). And Jose Baselga, last year sacked as president by Memorial Sloan Kettering Cancer Center, officially for hidden financial conflicts of interests (a “crime” many in academia commit with zero consequences, which makes the Baselga case rather unique). Former MD Anderson researcher researcher Dina Chelouche Lev (around a dozen papers on PubPeer) also dabbed in mTOR research, before she abruptly returned to Israel in 2013 to find a new faculty appointment 4 years later.
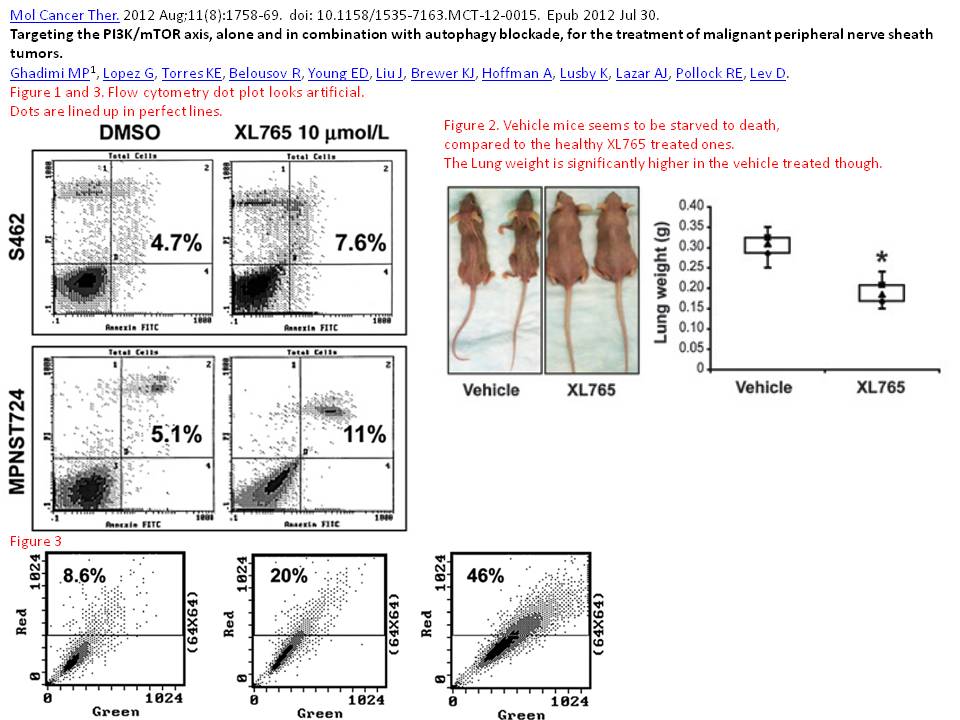
Markus P Ghadimi, Gonzalo Lopez, Keila E Torres, Roman Belousov, Eric D Young, Jeffery Liu, Kari J Brewer, Aviad Hoffman, Kristelle Lusby, Alexander J Lazar, Raphael E Pollock, Dina Lev Targeting the PI3K/mTOR axis, alone and in combination with autophagy blockade, for the treatment of malignant peripheral nerve sheath tumors Molecular cancer therapeutics (2012) doi: 10.1158/1535-7163.mct-12-0015
Mice “starved to death” to prove something about mTOR….
Another US-based Israeli, Count Fakula Michael Karin, of course also published about mTOR signalling. Then there was in that Pubpeer search a French researcher with penchant for self-introspection, Patrick Legembre, I wrote about him. Clare Francis pointed, among other names, to a paper by Amato Giaccia (whom I wrote about before), as well as to David Danielpour of Case Western Reserve University, who had one retraction for data manipulation and one embarrassing correction for his mTOR research contributions. Not to be forgotten is Mikhail Blagosklonny of Roswell Park Institute, editor of Oncotarget and Cell Cycle, who somehow has a Wikipedia page to educate the masses that:
“Blagosklonny has formulated a hypothesis about the possible role of TOR signaling in aging and cancer and proposed using rapamycin, a popular cancer drug as a possible treatment for life extension.[5] He advocates for rapamycin use in longevity research.[6]“
I discussed Blagosklonny’s questionable research in an article after his lawyer once tried to force me to love the fraud-peddling Oncotarget. Another mTOR enthusiast used to be the deceased Milanese cancer researcher Cesare Peschle, close collaborator of the godfather of fake cancer research, Carlo Croce. Peschle, described in the 2011 obituary as “father of studies of miRNA in tumours“, left behind a legacy of studies like this mTOR masterpiece:
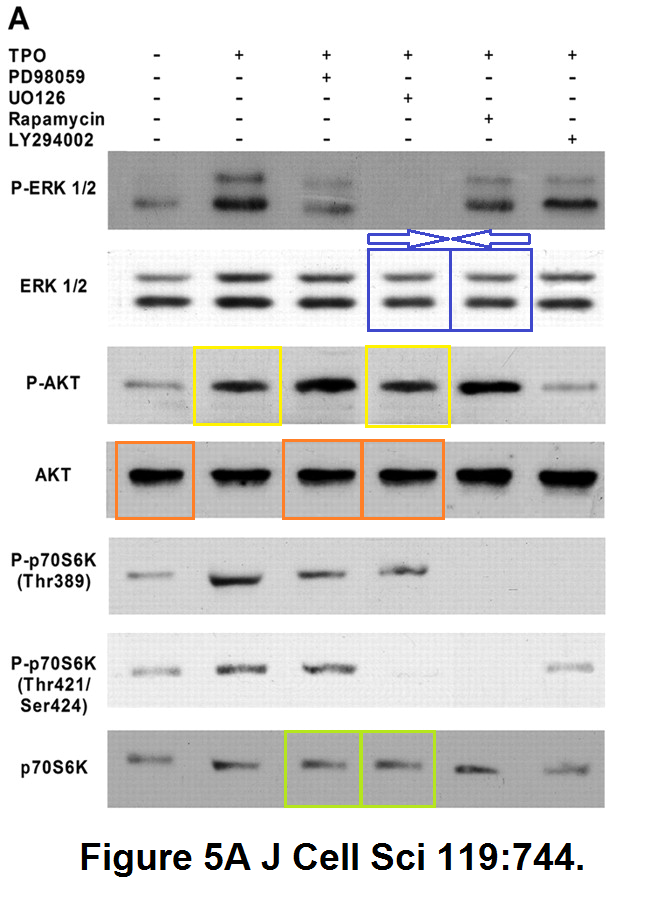
Raffaella Guerriero, Isabella Parolini, Ugo Testa, Paola Samoggia, Eleonora Petrucci, Massimo Sargiacomo, Cristiana Chelucci, Marco Gabbianelli, Cesare Peschle Inhibition of TPO-induced MEK or mTOR activity induces opposite effects on the ploidy of human differentiating megakaryocytes Journal of Cell Science (2006) doi: 10.1242/jcs.02784
In 2019, the Journal of Cell Science issued an eternally unresolvable Expression of Concern, a kind of tombstone for Peschle’s Pubpeer record, which went like this:
“After discussions with the first author, Raffaella Guerriero, who was unable to locate the original data, the journal referred this matter to the Istituto Superiore di Sanità. […] Following the investigation, the ISS Research Ethics Committee and the ISS Bioethics Unit confirm the results and conclusions of the paper by Guerriero et al. (2006).“
The conclusions are never affected because other dishonest scientists in the field published same fabricated junk. Now you know how the sausages are made.
And then of course also my most favourite of all scientists, Guido Kroemer, whom I believe to be some kind of metaphysical centre of the scientific dishonesty universe, contributed to the mTOR reserach area:
Maria Castedo, Thomas Roumier, Julià Blanco, Karine F Ferri, Jordi Barretina, Lionel A Tintignac, Karine Andreau, Jean-Luc Perfettini, Alessandra Amendola, Roberta Nardacci, Philip Leduc, Donald E Ingber, Sabine Druillennec, Bernard Roques, Serge A Leibovitch, Montserrat Vilella-Bach, Jie Chen, José A Este, Nazanine Modjtahedi, Mauro Piacentini, Guido Kroemer Sequential involvement of Cdk1, mTOR and p53 in apoptosis induced by the HIV-1 envelope The EMBO Journal (2002) doi: 10.1093/emboj/cdf391

Science gave up on Kroemer. He is free do do whatever he wants and no paper of his will be ever molested.
And this is how the mTOR field operates. To be fair, it’s not different from any other hot field of molecular signalling research.
But now you know why even results based on falsified figures are always scientifically reproducible, their conclusions not affected.
The article was supplemented with extra information after it was first published.
Donate!
If you are interested to support my work, you can leave here a small tip of $5. Or several of small tips, just increase the amount as you like (2x=€10; 5x=€25). Instead of wasting money to listen to these characters at conferences.
€5.00





“But now you know why even results based on falsified figures are always scientifically reproducible, their conclusions not affected”: not only mTOR but other big dogmas in science might be affected by this problem. Time to review a few important science dogmas.
LikeLike
I believe Choi’s toxic gas of choice is CO, not CO2.
LikeLike
Dang, will fix, thanks!
LikeLike
More crap from Weill-Cornell, eh? It appears that “:excellence” in photo shop extends out from the medical school dean here, who appears to be an excellent mentor and example to other labs in the school. So, his work on mentoring is well earned, but perhaps not in the way he had hoped.
Nor matter: These post-docs working for the mTOR leaders will get nice cushy faculty positions, writing grants to study mTOR which will get funded, because the leaders will read and approve the grants, as a way of validating their own “success”. An academic pyramid scheme, based on crap.
LikeLiked by 1 person
eg
https://www.nymc.edu/faculty/directory/by-name/holz-marina/
“”She is a dedicated mentor to students and laboratory scientists, with many of Dr. Holz’s trainees going on to successful health professional and biomedical research careers. “
LikeLike
Pingback: E sono tre - Ocasapiens - Blog - Repubblica.it
It’s like bad science is selected by natural selection
Michael Hall portfolio also includes:
https://pubpeer.com/publications/C5DF3C6693CDA0676256435167B40B
https://pubpeer.com/publications/AFCEE2C899AEA63DDB69D917583385
https://pubpeer.com/publications/B68002B1D3CA395EBEAAA69A32E1A2
Let’s not forget about Manning…
https://pubpeer.com/publications/5087C65F6A8AE8CAED7C361EDD7323#1
LikeLike
Thanks, added most of it to the article!
LikeLike
“Philippe P Roux, Bryan A Ballif, Rana Anjum, Steven P Gygi, John Blenis Tumor-promoting phorbol esters and activated Ras inactivate the tuberous sclerosis tumor suppressor complex via p90 ribosomal S6 kinase Proceedings of the National Academy of Sciences of the United States of America (2004) doi: 10.1073/pnas.0405659101”
“A bit of empty background and a pair of bands seem duplicated. Nothing sinister surely, probably there were antibody signals were the researchers did not expect any, and you know how this naughty S6K1 protein behaves. So the bands had to do as told as not to affect the mTOR conclusions.”
There does seem to be more. How to explain the background spots lining up after a horizontal flip?
Figure 5C.
https://pubpeer.com/publications/B5D8376A638154D162C0E277CF9CB1#4
https://pubpeer.com/publications/B5D8376A638154D162C0E277CF9CB1#5
LikeLike
Cell. 2010 May 14;141(4):632-44. doi: 10.1016/j.cell.2010.04.008. 2 comments on PubPeer (by: Pseudosinella Violeta, Aspalathus Villosa) Epub 2010 Apr 29.
Problematic data figure 2D. Much more similar than expected. See:https://imgur.com/Nd7i2xK
Transmembrane receptor DCC associates with protein synthesis machinery and regulates translationJoseph Tcherkezian 1, Perry A Brittis, Franziska Thomas, Philippe P Roux, John G Flanagan
Affiliation1Department of Cell Biology, Harvard Medical School, 240 Longwood Avenue, Boston, MA 02115, USA.PMID: 20434207
Problematic data figure 2D. Much more similar than expected.
LikeLike
Biochem J. 2012 Oct 1;447(1):159-66. doi: 10.1042/BJ20120938.
RSK phosphorylates SOS1 creating 14-3-3-docking sites and negatively regulating MAPK activation
Madhurima Saha 1, Audrey Carriere, Mujeeburahiman Cheerathodi, Xiaocui Zhang, Geneviève Lavoie, John Rush, Philippe P Roux, Bryan A Ballif
Affiliation1Department of Biology, University of Vermont, Burlington, VT 05405, USA.
PMID: 22827337 PMCID: PMC4198020 DOI: 10.1042/BJ20120938
Problematic data figure 1A. Much more similar than expected.
https://imgur.com/T37GD6CRSK
LikeLike
Cancer Res. 2014 Jan 1;74(1):201-11. doi: 10.1158/0008-5472.CAN-13-1175. Epub 2013 Nov 18.
Casein kinase 1ε promotes cell proliferation by regulating mRNA translation
Sejeong Shin 1, Laura Wolgamott, Philippe P Roux, Sang-Oh Yoon
Affiliation
1
Authors’ Affiliations: Department of Cancer and Cell Biology, University of Cincinnati College of Medicine, Cincinnati, Ohio; Department of Pathology and Cell Biology; and Institute for Research in Immunology and Cancer, Université de Montréal, Montreal, Quebec, Canada.
Problematic data figure 6. Much more similar and different than expected.
LikeLike
It appears that there are numerous retractions and EoCs for this field: http://retractiondatabase.org/RetractionSearch.aspx#?ttl%3dmTOR%2bOR%2brapamycin
LikeLike
Thanks for pointing that out.
This mTOR Expression of Concern is Harvard.
https://pubpeer.com/publications/5EBF91A44DDB8B2DC9D08C835DCE7B
https://retractionwatch.com/2019/10/15/cancer-lab-at-harvard-subject-of-inquiry/
Hot off the press!
https://retractionwatch.com/2020/11/24/former-harvard-cancer-researcher-faked-a-dozen-images-say-feds/
LikeLike
You ignorant rubes. Professor James Mier is entirely innocent. Look, David Panka did it. Harvard says so, ORI says so, confirmed by Retraction Watch.
That Panka is such a stealthy cunning crook, he managed to fake data in Mier lab papers, behind Prof Mier’s back, papers which Panka is not even coauthor on. LOCK HIM UP!!!!
https://pubpeer.com/publications/2BB990B82352FFE3F1842DFADC55C8
https://pubpeer.com/publications/58B4EA60743CE37821E5AC3B830D2F
LikeLike
Would it be fair to write that Harvard is the main swamp, and that Weill Cornell Medical College is over-spill?
In the mTOR field Lewis Cantley and John Blenis decamped from Harvard to Weill Cornell.
Most of the highly problematic mTOR papers in the current article were committed at Harvard (before the move).
LikeLike
I kind of want to become a famous person and insult Leonid, so that he would draw a picture of feces with flies for the top of my head in a picture. I would need a Sabitini-like hair-do to hold it in place, though. Sort of a turd toupee.
LikeLike
Proc Natl Acad Sci U S A. 2008 May 6;105(18):6584-9. doi: 10.1073/pnas.0802785105. Epub 2008 May 1.
Activation of PI3K/Akt and MAPK pathways regulates Myc-mediated transcription by phosphorylating and promoting the degradation of Mad1
Jidong Zhu 1, John Blenis, Junying Yuan
Affiliation1Department of Cell Biology, Harvard Medical School, Boston, MA 02115, USA.
Problematic data figure 5D. Mad1 panel does not look like it comes from the same blot as the other panels.
4 of the lanes in the Mad1 panel are much wider than the lanes in the other panels
https://imgur.com/5IfWdC5.
LikeLike
“Sang-Oh Yoon, Sejeong Shin, Yuzhen Liu, Bryan A. Ballif, Michele S. Woo, Steven P. Gygi, John Blenis Ran-binding protein 3 phosphorylation links the Ras and PI3-kinase pathways to nucleocytoplasmic transport Molecular Cell (2008) doi: 10.1016/j.molcel.2007.12.024”
More problematic data, this time figure 4A.
p-RanBP3 panel. Bands lanes 8 and 10 much more similar than expected.
P-Akt panel. Distribution signal bands lanes 4 and 7 much more similar than expected.
Pubpeer to date: https://pubpeer.com/publications/A95C507F1E494B2D4DB82FE2323A00
LikeLike
Yet more problematic data Mol Cell 2008 Feb 15;29(3):362-75. doi: 10.1016/j.molcel.2007.12.024.
This time figure 5F. Much more similar than expected after shifting lanes by one position and vertical re-resizing.
LikeLike
Another pioneer in “mTOR-world”, Enrique Rozengurt.
ULCA providing competition for Harvard!
Am J Physiol Gastrointest Liver Physiol 2005 Feb;288(2):G182-94. doi: 10.1152/ajpgi.00200.2004.
EGF receptor transactivation mediates ANG II-stimulated mitogenesis in intestinal epithelial cells through the PI3-kinase/Akt/mTOR/p70S6K1 signaling pathway
Terence Chiu 1, Chintda Santiskulvong, Enrique Rozengurt
Affiliation
1
Department of Medicine, School of Medicine, CURE, Digestive Diseases Research Center, Molecular Biology Institute, University of California, Los Angeles, California, USA.
PMID: 15358595 DOI: 10.1152/ajpgi.00200.2004
Problematic data figure 1C. Much more similar than expected.
Problematic data figure 3. Much more similar and different than expected.
Endocrinology. 2007 Jul;148(7):3246-57. doi: 10.1210/en.2006-1711. Epub 2007 Mar 22.
Insulin potentiates Ca2+ signaling and phosphatidylinositol 4,5-bisphosphate hydrolysis induced by Gq protein-coupled receptor agonists through an mTOR-dependent pathway
Krisztina Kisfalvi 1, Osvaldo Rey, Steven H Young, James Sinnett-Smith, Enrique Rozengurt
Affiliation
1
Unit of Signal Transduction and Gastrointestinal Cancer, Department of Medicine, David Geffen School of Medicine, University of California at Los Angeles-Center for Ulcer Research and Education, Los Angeles, California 90095-1786, USA.
Problematic data figure 5A. Much more similar than expected.
LikeLike
“The secret of success is constancy to purpose”. Benjamin Disraeli.
PLoS One. 2013;8(2):e57289. doi: 10.1371/journal.pone.0057289. Epub 2013 Feb 21.
Different patterns of Akt and ERK feedback activation in response to rapamycin, active-site mTOR inhibitors and metformin in pancreatic cancer cells
Heloisa P Soares 1, Yang Ni, Krisztina Kisfalvi, James Sinnett-Smith, Enrique Rozengurt
Affiliations collapse
Affiliation
1Division of Digestive Diseases, Department of Medicine; CURE: Digestive Diseases Research Center David Geffen School of Medicine and Molecular Biology Institute, University of California at Los Angeles, Los Angeles, California, United States of America.
PMID: 23437362
Pubpeer 6 years ago! https://pubpeer.com/publications/439990EB773671BEFD33CF06F9A179
LikeLike
Trail blazer in “signal transdudction”, Enrique Rozengurt!
J Biol Chem. 1996 Nov 15;271(46):29453-60. doi: 10.1074/jbc.271.46.29453.
[D-Arg1,D-Trp5,7,9,Leu11]Substance P coordinately and reversibly inhibits bombesin- and vasopressin-induced signal transduction pathways in Swiss 3T3 cells
M J Seckl 1, T Higgins, E Rozengurt
Affiliations collapse
Affiliation
1Imperial Cancer Research Fund, London WC2A 3PX, United Kingdom.
PMID: 8910612
Probleamatic data figure 4.
Pubpeer 6 years ago: https://pubpeer.com/publications/C4A38F058601D3B951042F2B964D98#
First author has a 2015 retraction of a 2002 J Biol Chem paper.
https://retractionwatch.com/2015/06/30/jbc-cancer-paper-felled-by-duplication-is-one-authors-second-retraction-this-month/
LikeLike
Another contribution to cell signalling!
J Biol Chem. 2005 Jun 24;280(25):24212-20. doi: 10.1074/jbc.M500716200. Epub 2005 Apr 21.
G protein-coupled receptor activation rapidly stimulates focal adhesion kinase phosphorylation at Ser-843.
Mediation by Ca2+, calmodulin, and Ca2+/calmodulin-dependent kinase II
Robert S Fan 1, Rodrigo O Jácamo, Xiaohua Jiang, James Sinnett-Smith, Enrique Rozengurt
Affiliation1
Division of Digestive Diseases, Department of Medicine, David Geffen School of Medicine, Center for Ulcer Research and Education, Digestive Diseases Research Center and Molecular Biology Institute, University of California, Los Angeles, California, USA.
PMID: 15845548DOI: 10.1074/jbc.M500716200
Problematic data figures 7B and 7C. Much more similar than expected.
LikeLike
Pushing the boundaries of science again.
Am J Physiol Gastrointest Liver Physiol. 2008 Dec;295(6):G1190-201. doi: 10.1152/ajpgi.90452.2008. Epub 2008 Oct 9.
Protein kinase D1 mediates NF-kappaB activation induced by cholecystokinin and cholinergic signaling in pancreatic acinar cells
Jingzhen Yuan 1, Aurelia Lugea, Ling Zheng, Ilya Gukovsky, Mouad Edderkaoui, Enrique Rozengurt, Stephen J Pandol
Affiliation
1
Veterans Affairs Greater Los Angeles Healthcare System, West Los Angeles VA Healthcare Center, Los Angeles, CA 90073, USA. jzyuan@ucla.edu
PMID: 18845574 PMCID: PMC2604803 DOI: 10.1152/ajpgi.90452.2008
Problematic data figure 3B. Much more similar than expected.
Senior author is on editorial board of the journal.
https://journals.physiology.org/ajpgi/edboard
Stephen J. Pandol
Cedars-Sinai Medical Center, Los Angeles, California
Stephen J. PandolStephen J. Pandol, M.D. Dr. Stephen Pandol is the director of basic and translational pancreatic research at Cedars-Sinai Medical Center. He is a noted expert in pancreatic cellular physiology and mechanism of the disease. In his position he is responsible for organizing research efforts to develop potential therapies for pancreatic diseases, including pancreatitis (inflammation of the pancreas), pancreatic cancer, and diabetes. The research efforts encompass projects to determine basic mechanisms of disease and projects using this information to develop new therapies including prevention. The team of researchers include scientists with skills chemistry and biology to those conducting clinical trials in patients using new agents developed by the group.
LikeLike
Why I’m still in this dirty, money grubbing business called academic scientific research is beyond me.
LikeLike
Stephen J. Pandol “The team of researchers include scientists with skills chemistry and biology to those conducting clinical trials in patients using new agents developed by the group.”
I think they should add “photo-shopping”, or is that a form of magic?
J Biol Chem. 2011 Mar 11;286(10):7779-87. doi: 10.1074/jbc.M110.200063. Epub 2010 Nov 30.
NADPH oxidase activation in pancreatic cancer cells is mediated through Akt-dependent up-regulation of p22phox
Mouad Edderkaoui 1, Claudia Nitsche, Ling Zheng, Stephen J Pandol, Ilya Gukovsky, Anna S Gukovskaya
Affiliation
1Veterans Affairs Greater Los Angeles Healthcare System and UCLA, Los Angeles, California 90073, USA.
PMID: 21118808 PMCID: PMC3048665 DOI: 10.1074/jbc.M110.200063
Problematic data figure 3B. Much more similar than expected.
Problematic data figure 1B. Much more similar than expected.
LikeLike
Lol! A very slick primer on detecting data fraud from a rapidly talking ASU presenter; Fun to watch. Leave it to ASU, trying to make themselves look a little better:
LikeLike
J Biol Chem. 2005 Oct 28;280(43):35829-35. doi: 10.1074/jbc.M504192200. Epub 2005 Aug 18.
mTOR-dependent suppression of protein phosphatase 2A is critical for phospholipase D survival signals in human breast cancer cells
Li Hui 1, Vanessa Rodrik, Rafal M Pielak, Stefan Knirr, Yang Zheng, David A Foster
Affiliations collapse
Affiliation
1Department of Biological Sciences, Hunter College of the City University of New York, New York, New York 10021, USA.
PMID: 16109716
DOI: 10.1074/jbc.M504192200
Figure 2A. Much more similar than expected.
Figure 1. Much more similar than expected.
LikeLike
Cancer Discov. 2011 Aug;1(3):248-59. doi: 10.1158/2159-8290.CD-11-0085. Epub 2011 Jun 17.
mTOR kinase inhibition causes feedback-dependent biphasic regulation of AKT signaling
Vanessa S Rodrik-Outmezguine 1, Sarat Chandarlapaty, Nen C Pagano, Poulikos I Poulikakos, Maurizio Scaltriti, Elizabeth Moskatel, José Baselga, Sylvie Guichard, Neal Rosen
Affiliation
1Program in Molecular Pharmacology and Chemistry, Memorial Sloan-Kettering Cancer Center, New York, New York 10065, USA.
PMID: 22140653 PMCID: PMC3227125 DOI: 10.1158/2159-8290.CD-11-0085
Figure 2. Much more similar than expected.
One of the two Editors-in-Chief Cancer Discov is Lewis C Cantley.https://cancerdiscovery.aacrjournals.org/site/misc/editors.xhtml
Editors-in-Chief
Lewis C. Cantley
Luis A. Diaz
One of the authors, José Baselga, was the subject of articles by the New York Times.https://www.nytimes.com/2018/09/08/health/jose-baselga-cancer-memorial-sloan-kettering.htmlhttps://www.nytimes.com/2019/01/07/health/baselga-sloan-kettering-astrazeneca.htmlhttps://www.nytimes.com/2020/12/22/health/memorial-sloan-kettering-cancer-center-doctor-jose-baselga.html
LikeLike
You need a space between the two Urls you posted at the end. Right now it’s reading as only a single address, so the link doesn’t work.
LikeLike
J Cell Biol. 2003 Oct 27;163(2):315-26. doi: 10.1083/jcb.200304159. Epub 2003 Oct 20.
Akt activation disrupts mammary acinar architecture and enhances proliferation in an mTOR-dependent manner
Jayanta Debnath 1, Stephanie J Walker, Joan S Brugge
Affiliation1Department of Cell Biology, Harvard Medical School, Boston, MA 02115, USA.PMID: 14568991 PMCID: PMC2173511 DOI: 10.1083/jcb.200304159
Problematic data figure 4A. Much more similar than expected.
LikeLike
Mol Cell. 2006 Oct 20;24(2):185-97. doi: 10.1016/j.molcel.2006.09.019.
S6K1 regulates GSK3 under conditions of mTOR-dependent feedb…S6K1 regulates GSK3 under conditions of mTOR-dependent feedback inhibition of Akt
Hui H Zhang 1, Alex I Lipovsky, Christian C Dibble, Mustafa Sahin, Brendan D Manning
Affiliations collapseAffiliation1Department of Genetics and Complex Diseases, Harvard School of Public Health, Harvard Medical School, Boston, Massachusetts 02115, USA.
PMID: 17052453 PMCID: PMC1880887 DOI: 10.1016/j.molcel.2006.09.019
Problematic data figure 2C. Much more similar after horizontal flipping than expected (twice).
I came across this independently, but somebody else has noticed the same problems with the data 5 months ago.
https://pubpeer.com/publications/5087C65F6A8AE8CAED7C361EDD7323
LikeLike
Additional problematic data Mol Cell. 2006 Oct 20;24(2):185-97. doi: 10.1016/j.molcel.2006.09.019.
Problematic data figure 4E. Much more similar than expected.
LikeLike
Is Brendan Manning related to Bernard Manning?
LikeLike
Brendan Manning, Harvard.
“This change does not alter the results or conclusions that can be drawn from this experiment or any other in this manuscript. ” For ever, and ever. Amen”
S6K1 regulates GSK3 under conditions of mTOR-dependent feedback inhibition of Akt
Molecular Cell (2006) – 6 Comments
pubmed: 17052453 doi: 10.1016/j.molcel.2006.09.019 issn: 1097-2765
Hui H. Zhang author has email , Alex I. Lipovsky , Christian C. Dibble author has email , Mustafa Sahin author has email , Brendan D. Manning
https://pubpeer.com/publications/5087C65F6A8AE8CAED7C361EDD7323#6
September 2023 correction.
https://www.cell.com/molecular-cell/fulltext/S1097-2765(23)00616-0
”In the original published version of this article, the GSK3β and S6K1 blots in Figure 2C, serving as total protein controls for the corresponding phospho-blots, were erroneously shown as duplicated and inverted images of the same blot for the vector and TSC2-WT cell lines in the composite. This inadvertent error in assembling the composite stemmed from the way the blots were exposed on the same film as mirror images of themselves. The corrected composite for Figure 2C is shown here. The error does not alter the results or conclusions that can be drawn from this experiment or any other in this manuscript. The authors regret this error. Questions have risen about the similarity of two phospho-GSK3β bands in Figure 4E, which is apparent in some versions of the original Figure 4. The authors were unable to find the original developed film representing that original panel but were able to identify a blot from the same experiment. Thus, a newly made composite of this experiment is shown here. This change does not alter the results or conclusions that can be drawn from this experiment or any other in this manuscript. The authors regret this error.”
LikeLike