The owners of the UK-based degenerative medicine company Celixir are rightfully scared of Dr Patricia Murray, Professor of Cellular and Molecular Physiology at the University of Liverpool, UK. After the initial article on my site, Murray published a detailed investigation of Celixir’s questionable activities, please refer to those for the background of this case. After the initial shock and after having fetched a fresh pair of trousers, the Celixir CEO Ajan Reginald and his colleagues now formulated a reply to Murray’s criticisms, hoping it will fool journalists and authorities. The full Celixir document is available here.
Murray now publishes a rebuttal to Celixir’s rebuttal, again here on my site. It follows below, after this summary I made.

What transpires from Murray’s investigations, to me at least, is the following (though Celixir half-heartedly denies it):
- In 2009, the British star surgeon and self-proclaimed psychopath Stephen Westaby went to Thessaloniki, Greece to collaborate with his former mentees on a clinical trial. The idea was to use myocardial infarction (MI) patient’s own blood cells for heart regeneration by injecting those into their own heart, under the harebrained assumption that the blood of MI patients is somehow primed by a magic healing power. Crazy, and very dangerous, but those were the crazy and dangerous days of stem cell mavericks like Paolo Macchiarini or, closer to the field, Bodo Strauer.
- In same year 2009, the company Celixir (then called Cell Therapy Ltd) was founded in Wales by Sir Martin Evans, Nobel Prize winner for stem cell discoveries and former President of Cardiff University, and the struck-off dentist Ajan Reginald, a greedy scam artist and a pathological liar. Westaby joined the Celixir board, while Celixir sought to market that magic blood which can cure broken hearts. Yet autologous cells to be used in the same patient are not patentable, so what then?
- In comes Ina Laura Pieper, a PhD student from Sweden. The Swansea University in Wales is apparently a circus of criminal clowns, hence Pieper was given a public grant to do a PhD for Celixir on “mesenchymal stem cell therapy for the treatment of myocardial infarction”. In 2011, the first-year PhD student and Celixir employee was appointed principal investigator of a clinical research project at the affiliated Morriston Hospital. There, MI patients were requested to donate blood for Pieper’s unconvincingly outlined “research”, while agreeing for their blood to be used for commercial purposes. The entire Celixir gang, Ajan, Pieper, Sir Martin and “Global Head of Research” Sabena Sultan took up business residence at Swansea University.
- The blood of MI patients was eventually patented by Celixir. You may wonder how that works, legally? Simple: to claim novelty, Celixir plagiarised data from unrelated research in USA to bullshit the patent office those cells can specifically home onto diseased tissues, after a magic reformulation as alleged bone marrow cells. Obviously the flow cytometry profiles of peripheral blood and bone marrow cells are very different, and that, along with plagiarised data, sufficed to convince the experts at European Patent Office in Munich. The technology was then licenced for £12.5 million to a Japanese company.
- In 2012, a phase 2a clinical trial took place in Thessaloniki, on 11 patients. Most likely the blood from Morriston patients was used, back then it was described as “mesenchymal stem/progenitor cells”, but this cell type lost its magic appeal since (what with all the fraud around it). This was why Celixir recently retrospectively renamed the cells as iMPs (whatever that is) and insists those are never ever mesenchymal stem cells. Indeed, they are most likely just some blood.
- Now, Celixir received approval from the British Medicines and Healthcare products Regulatory Agency (MHRA) to perform a phase 2b clinical trial at the Royal Brompton hospital in UK. Which cells will they use? There are clues. Celixir namely set up in 2016 a GMP facility to produce iMP cells in Thessaloniki. Will the hapless Greek MI patients be bled now and 50 British MI patients experimented upon, so Ajan and Sir Martin get rich? Quite likely. In the event of hard Brexit, this route won’t be available though, but Ajan already made contact with a US company AllCells which sells cells.
The only person willing to stop this dangerous travesty is Professor Murray of University of Liverpool. It would be nice if other academics had a shred of her civil courage and integrity, and speak out also. Is MHRA willing to reconsider its approval for Celixir, and choose patient safety over business interests? Is it ****. MHRA’s approval for Macchiarini-style trachea transplants to go ahead is still standing, in case you wondered. After all that investigative work Murray did in that regard, and the legal threats she received.

The questionable activities of the UK company Celixir: response to Celixir’s rebuttal
By Patricia Murray
Celixir has not adequately addressed the issues raised in my previous report, and their rebuttal raises further questions. Moreover, documents released by the MHRA (under FOIA) regarding Celixir’s application for Clinical Trials Authorisation (CTA) raise additional concerns about the Company’s activities.
Celixir claim that the use of plagiarised data in one of their patents was a “genuine mistake” due to a “referencing error”. It is not possible to copy and paste data from another research group and insert it into one’s own document ‘by mistake’.
This issue, along with additional scientific concerns, are discussed below.
1. Background relating to Celixir’s clinical trials
Celixir has confirmed that they used their immunomodulatory progenitor cells (iMPs) in the 2012-2013 Greek trial, but they have not addressed the additional questions raised in my report:
- Why did Celixir retrospectively replace ‘MSCs’ with ‘iMPs’ in May 2019, several years after the trial’s completion data?
- Why did Celixir retrospectively change the primary and secondary endpoints several years after the trial’s completion date?
- What data had Celixir obtained regarding the properties of the iMPs prior to their use in the Greek trial (i.e., prior to November 2012)?
- What data had Celixir obtained regarding the preclinical safety and efficacy of the iMPs prior to their use in the Greek trial (i.e, prior to November 2012)?
2. Questions relating to Celixir’s proprietary cell type ‘iMPs’
2.1 Although Celixir emphasise in their patents that the ‘iMPs’ and ‘progenitor cells of mesodermal lineage’ (PMLs) are not mesenchymal stromal cells(MSCs), their rebuttal document confirms that they had initially referred to them as ‘peripheral blood-derived mesenchymal stromal cells’ (PB-MSCs). They state: “When filing the PML and iMP patent applications, we chose to rename the two PB-MSC cell types as PMLs and iMP cells.”
It is difficult to reconcile these inconsistencies.
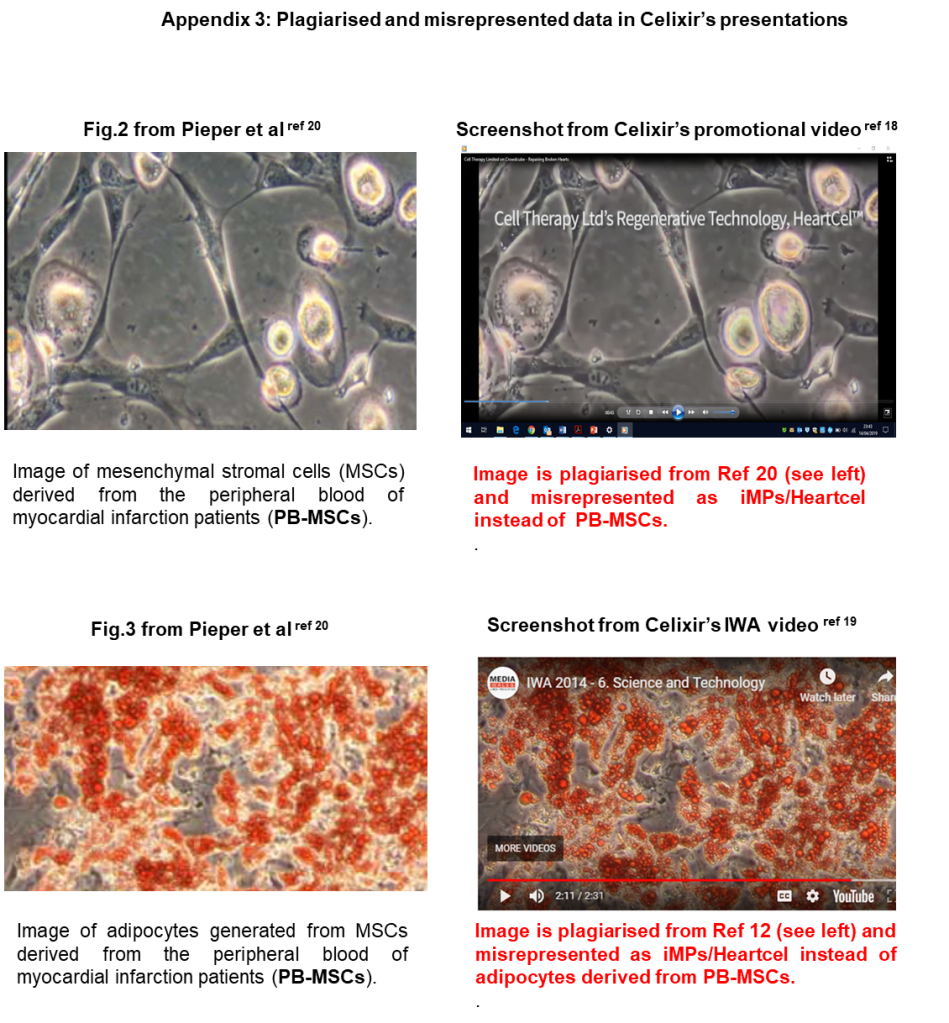
2.2 Celixir state that the use of Ina Laura Pieper’s data in their patents and promotional material “cannot represent plagiarism”. However, the point made in my report was not so much that Pieper’s data was used, but rather that is was misrepresented by Celixir. Pieper’s 2017 paper makes it clear that she was only able to isolate PB-MSCs from patients who had been admitted to the Morriston Hospital following a myocardial infarction (MI; aka ‘heart attack’). Of note, she was unable to isolate PB-MSCs from healthy volunteers:
“This pilot study demonstrates for the first time that MSCs…… can be isolated and cultured from the peripheral venous blood of STEMI patients [the Morriston MI patients], but not from healthy volunteers”. [Pieper et al 2017]
What this means is that the cells that Celixir were calling ‘iMPs’ and ‘PMLs’ were actually PB-MSCs isolated from MI patients at the Morriston hospital, not healthy volunteers.
2.3 Celixir state: “We could not go back in time and remove previous references to stem cells and have struggled to prevent third parties from using such a common and well-recognised term.”
Celixir themselves refer to these cells as ‘stem cells’ in their promotional videos and press releases.
For example, in a promotional video, Sabena Sultan states: “This is CTL’s proprietary heart-specific stem cell, ‘HeartCel’ (aka iMPs).”
2.4 Celixir state they are confident that PMLs and iMPs are different from one another because:
- production of iMP cells takes longer (15-30 days) than that of PMLs (14 days or less)
- the different production methods result in different phenotypes
- they have different marker expression profiles
As indicated in my report, the protocols for PMLs and iMPs in the respective patents are identical.
From the PML patent:
“step (a) of the method of the invention comprises culturing MCs in a medium comprising platelet lysate for sufficient time to induce the MCs to differentiate into progenitor cells of mesodermal lineage. The sufficient time is typically from about 15 to about 25 days, preferably about 22 days.”
From the iMP patent:
“step (a) of the method of the invention comprises culturing MCs in a medium comprising platelet lysate for sufficient time to induce the MCs to differentiate into IMP cells. The sufficient time is typically from about 15 to about 25 days, preferably about 22 days.”
The production methods are identical and there is no evidence for different phenotypes. There are no comparisons of marker expression profiles between the so-called PMLs and iMPs.

2.5 Celixir state: “PMLs and iMP cells are not MSCs. They are not produced using standard protocols for isolating MSCs. We have reviewed dozens of references relating to MSC isolation and none of them disclose our method of culturing MNCs [mononuclear cells] in our proprietary, supplemented culture medium.”
On the contrary, the protocols in Celixir’s PML and iMP patents are standard MSC isolation protocols. In the initial patents there is no mention of proprietary supplemented culture medium.
This view is entirely consistent with that of the European Patent Office, whose report from January 2014 stated that all 38 claims in the initial version of the PML patent were refused due to both lack of novelty and lack of any inventive steps. As indicated in the following excerpts, the report makes it absolutely clear that the cells described in the patent are MSC:
“Novelty claims 1-38 cannot be considered novel”
“Claim 1 defines the cells as typical mesenchymal stem cells as known in the prior art…[..]…The dependent claims try to further characterise the cells in sub-populations that express further markers.
“Thus the cells claimed are seen as MSCs that according to different factors may acquire the phenotype required by the claims. The cells claimed cannot be distinguished from the MSCs of the prior art…”
“The prior art documents [i.e., previous literature in the field of MSC biology] do not analyse all the markers listed in the application. This, however, does not mean that the cells claimed are different from those described in the prior art…..It does not seem that the claimed cells have any special feature that may clearly distinguish them from known MSCs.”

The PML patent was eventually granted in March 2018 after the claims were reduced down to 15. However, one of remaining claims is that the PMLs can migrate to damaged tissue (specifically, heart, retinal or bone tissue), and the data used to back-up this claim are the data that have been plagiarised and misrepresented from the US group.
2.6 Data from US group which are plagiarised and misrepresented in Celixir’s patent
Celixir claim that this was a “genuine mistake” due to a “referencing error”.
This is implausible. It is not possible to copy and paste data from another research group and insert it into one’s own document ‘by mistake’. Furthermore, not only did Celixir copy and paste the data but they also re-named the cell type. So what were labelled as ‘MSCs’ in the US paper became ‘progenitor cells of mesodermal origin’ (PMLs) in Celixir’s patent. Importantly, in the text of the patent, they describe these results as being their own data. How can Celixir claim that this was a “genuine mistake” due to a “referencing error”?
Celixir claim: “removal of the data in Figure 5 from the PML patents/applications does not affect the patentability of the PMLs”.
This statement is incorrect. The copied and modified data from the US group are used in the PML patent to back-up Celixir’s claim that the PMLs can home to injured heart and bone tissue. In the granted PML patent, claim 3 is as follows:
“A progenitor cell according to claim 2, wherein the specific tissue is;
- heart tissue or bone tissue and the cell expresses detectable levels of C-X-C chemokine receptor type 4 (CXCR4).”
To back-up this claim, the main text of the patent includes the following statement:
“Cells capable of homing to damaged heart tissue and bone tissues were shown to express CXCR4. Fig.5 shows that the CXCR4 positive cells are capable of homing to damaged bone”.
Fig.5 comprises the data that have been plagiarised and misrepresented from the US group. Celixir do not appear to have any of their own data to show that their cells are capable of homing to damaged tissue.
3. Issues relating to trial NCT01753440, undertaken in Greece in 2012-2013
3.1 Source of cells used in the Greek 2012-2013 trial
3.1.1 Celixir state: “The PB-MSCs produced by Dr Pieper from Welsh donors were not used in the Greek study. The study used iMP cells produced in an accredited Greek laboratory [citing Anastasiadis et al, 2016, the paper reporting on the Greek trial].
This statement is not consistent with the information provided in the Anastasiadis et al 2016 paper. The paper states the following:
“iMPs are a novel and distinct mesenchymal precursor cell type discovered and isolated by Cell Therapy Limited, UK (CTL)”
The paper does not mention that an accredited Greek laboratory was used.
The 2016 paper claims that the cells were obtained from the bone marrow of a healthy donor, but no information is provided regarding the recruitment of this donor. Which authority in Greece granted the ethical approval for this procedure? Who aspirated the bone marrow?
The paper acknowledges an individual called Nancy Piouka for assistance with preparing the cells. Piouka’s Linkedin page indicates she was not recruited by Celixir until 2013. The trial started in November 2012. Who prepared the cells for the first patient?

3.1.2 Celixir state: “We initially isolated PMLs and iMP cells (called PB-MSCs at the time) from peripheral blood. The peripheral blood iMP cells (PB-MSCs) were analysed and, as noted, the data formed the basis of the iMP patent application. Before the Greek study, we switched to using bone marrow MNCs [mononuclear cells] to tissue engineer the iMP cells.”
In November 2011, Ina Laura Pieper applied to the South West Wales Research Ethics Committee (REC) for an amendment to Celixir’s ethics application. The main changes she requested included increasing the sample size of the Morriston MI patients (the peripheral blood donors), and increasing the volume of blood drawn on each occasion from 18ml to 20ml.
The reasons given for increasing the number of MI patients was as follows:
“…when the cells presented themselves in culture [the cells from the peripheral blood of the MI patients], I had to empirically learn how to care for them to get them so survive. The first patient samples were therefore used to develop a standardised procedure for how to culture them. More research will need to go into how to optimise their growth…”
“Therefore I would like to request to increase the sample size…..I would then “re-start” with the future patient batches and treat all the cell samples in the same standardised manner, so that they are comparable.”
Pieper’s request for amendment makes it clear that by November 2011, she had only established how to isolate cells from the peripheral blood of the MI patients, and required additional samples to investigate how to optimise their growth. The granted amendment was dated 15th December 2011.

If Celixir’s statement is correct, this would mean that between December 2011 and November 2012 (the date the first patient in the Greek trial was treated), Celixir would have achieved the following:
- optimised culture conditions to promote the growth of the peripheral blood-derived cells;
- fully characterised the cells, including determining their proliferation rate, differentiation potential, karyotype and surface marker expression (via flow cytometry);
- implemented a protocol for isolating mononuclear cells (MNCs) from human bone marrow samples (obtained from orthopaedic patients attending the Morriston hospital) and differentiating them into ‘iMPs’;
- fully characterised the bone marrow-derived cells as above and compared their properties with the peripheral blood-derived cells;
- established the protocol for isolating the cells from bone marrow in an accredited Greek facility under GMP conditions (which facility, and from which volunteers?)
- undertaken animal tests to ensure that the cells were likely to be safe.
It is unrealistic to suggest that all of the above activities could have been satisfactorily completed within 12 months.
3.2 Celixir state they have “extensive data confirming we can make the same iMP cells from both sources [i.e., peripheral blood and bone marrow]”
No such data are provided.
3.3 Celixir state: “The 2016 Anastasiadis paper confirms the iMP cells used in the Greek study were derived from bone marrow. Table 1 of the 2016 paper is taken from the patent application (which used peripheral blood derived iMP cells/PB-MSCs) as the data are representative of iMP cells and the paper does not state the data relate to bone marrow derived iMP cells.”

Firstly, the 2016 paper does not confirm that the iMP cells were derived from bone marrow; Celixir simply state this to be so, but no evidence is provided.
Celixir then admit that although they claimed that the cells used in the trial were from bone marrow, the data they show in Table 1 of the paper relate to peripheral blood-derived mesenchymal stromal cells (PB-MSCs).
Celixir say they do not state in the 2016 paper that the flow cytometry data in Table 1 relate to bone marrow cells. Nevertheless, anyone reading the paper would form that view because the term ‘peripheral blood’ is not mentioned anywhere in the paper. The purpose of the flow cytometry data was to demonstrate that Celixir’s iMPs were unique and had a distinct surface marker expression profile compared with proprietary bone marrow-derived MSCs. However, the fact that the iMPs were derived from peripheral blood rendered the flow cytometry data meaningless; cells derived from peripheral blood would be expected to have a different surface marker expression profile than cells derived from bone marrow, especially when the peripheral blood has been obtained from patients who had recently had an MI (see section 2.2).
If Celixir had really used bone marrow as the source of iMPs in the Greek trial (rather than peripheral blood), why didn’t they show flow cytometry data from the bone marrow-derived cells?
3.4 Celixir state: “The videos [their promotional videos] never stated these cells were derived from bone marrow”.
Celixir’s videos[here and here] misrepresented cells that had been isolated from the peripheral blood of patients admitted to the Morriston Hospital following a myocardial infarction as unique stem cells that were capable of regenerating cardiac tissue.
Celixir state: “We cannot see how this [the fact that they used peripheral blood cells from the Morriston patients] contradicts the clear statement in the peer-reviewed 2016 Anastasiadis paper that the cells were derived from bone marrow.”
These claims are contradictory.
3.5 Celixir state: “We did in 2011 amend our REC application concerning the Welsh PB-MSCs to refer to therapeutic use, but this was to keep our options open (as was our prerogative).”
Celixir state that they were considering using the PB-MSCs from the Morriston MI patients as therapies in the Greek trial, but go on to state that they instead used cells from the bone marrow of a healthy donor manufactured by “an accredited Greek laboratory”. Yet, no such laboratory is mentioned in the 2016 paper that reported on the Greek trial.
3.6 Celixir state: “The peer reviewers agreed that the IMPs “improved myocardial contractility and perfusion of nonrevascularized territories resulting in a significant reduction in left ventricular scar area at 12 months after treatment” (abstract)”.
This highlights a shortcoming in the review process in that it failed to correct this misleading claim. All trial patients had coronary artery bypass grafting (CABG) and cell injection (i.e., there was no control group), so it is not possible to claim any benefit of the cell injection as it would be expected that improvements would occur as a result of CABG.
3.7 Question regarding affiliations
Celixir state: “We are comfortable with the affiliations of the co-authors in the 2016 Anastasiadis paper. Ajan Reginald was studying for a MSc in Experimental Therapeutics at the University of Oxford at the time of publication and matriculated in 2014 and graduated in 2017.”
Ajan Reginald indicated his affiliation was ‘Experimental Therapeutics’, University of Oxford, when he was actually studying for a part-time MSc in the Department of Continuing Education at the University of Oxford. If Ajan Reginald matriculated in October 2014, he should not have indicated he was affiliated to the University of Oxford because the Greek trial was completed prior to this date.

It is also worth noting that despite previously apologising for calling himself a doctor in a media report published in August 2012, Ajan Reginald was again referring to himself as ‘Dr Reginald’ in documents submitted to Companies House in 2016, and is currently being presented online as a London-based medical doctor. He also appears to have falsely presented himself as having an MD degree on a research publication. Celixir have provided no explanation for this.

Celixir have not explained why the three co-authors, Reginald, Sultan and Evans, gave false affiliations. Documents concerning the iMP patent that were submitted to the European Patent Office (EPO) state that the address of the three co-authors was in Swansea:
“The Applicant is Cell Therapy Limited, Institute of Life Sciences, First Floor, Room 137, School of Medicine, Swansea University, Singleton Part, Swansea SA2 8PP. The inventors’ names and addresses are given in the PCT Request.”

The inventors are Reginald, Sultan and Evans, and their addresses are all identical to the Swansea address indicated above with postcode SA2 8PP. Importantly, the above document was submitted to the EPO one month after the paper reporting on the trial (Anastasiadis et al, 2016) was first submitted for publication.

3.8 Celixir state that the Greek co-authors chose to cite a Cochrane Review rather than a paper describing their previous clinical trial.
The previous trial (undertaken in 2009-2011) was identical to the 2012-2013 Greek trial except that in the previous trial, the patients’ own bone marrow-derived cells were used. This paper should have been cited and results compared between the two studies. It is very odd that the Greek co-authors chose not to cite their own paper.
Celixir state: “It is also noteworthy that the earlier study did not suggest scar reduction following treatment. The earlier Greek study also used autologous cells.”
This statement is misleading. Firstly, it is important to point out that the degree of scar tissue was not directly measured in either the 2009-2011 or 2012-2013 Greek studies. Rather, a single photon emission computer tomography (SPECT) imaging technique was used to measure the uptake of a thallium radioactive tracer. The degree of thallium uptake indicates the degree to which the heart is perfused. Both studies showed there was an increase in thallium uptake following the injection of either the autologous bone marrow-derived cells or the ‘iMPs’ at the time of CABG. Importantly, however, it has been demonstrated that thallium uptake also increases after CABG alone. The following statement is from a research paper published in 1983, showing an increase in thallium uptake after CABG alone (i.e., without additional cells).
“Of 42 persistent defects thought to represent myocardial scar before surgery, 19(45%) demonstrated normal perfusion postoperatively.” [Gibson et al 1983]
It is therefore not possible to claim that either cell type reduced scarring in the heart.
4. Issues relating to the forthcoming trial NCT03515291 at the Royal Brompton Hospital
4.1 What modifications are applied to the cells in Celixir’s Greek facility?
As indicated in my previous report, Celixir’s CEO has previously stated that the cells were “modified” to make them “heart-specific” and then “modified” again to make them “very good at reducing scar in the heart.”
Celixir now state: “The iMP cells are modified because the cells change from MNCs [mononuclear cells] to iMP cells”.
As indicated in section 2.5 above, iMP cells appear to be MSCs. Therefore, it seems that no specific modifications are planned in the Greek facility, and that the standard protocol described in Celixir’s patents for deriving MSCs from bone marrow-derived MNCs will be used [here and here].
It isn’t clear from the documents provided by the MHRA, whether Celixir are planning to purchase bone marrow or bone marrow-derived MNCs from AllCells, nor is it clear why the bone marrow is being sourced from a US company. Why don’t Celixir use the same source of bone marrow that they allegedly used in the Greek trial?
5. Celixir’s subsidiary companies, funders and supporters
Questions regarding the appropriateness of Celixir’s promotional video
It is important to note that the Greek trial recruited 11 patients between November 2012 and September 2013. Each patient was followed up for 1 year. This means that the trial results would not have been available until September 2014 at the earliest. Yet, by January 2014, according to a BBC news article, Celixir were already claiming their trial was successful.
The BBC report, which provides the introduction to the promotional video, contains some very misleading quotes from the CEO, Ajan Reginald:
“We’ve identified what we think is a very potent type of stem cell which is heart specific.”
Note the use of the term stem cell, despite the fact that in their patents, Celixir insist that the cells are not stem cells (see section 2.3).
The claim that the cells are ‘heart specific’ is unsubstantiated.
“We’ve finished our first clinical trial which was focused on safety. The interim analysis looks very positive and very fortunately we’ve also seen some benefit – the study does show some signs of early regeneration.”
In January 2014, the trial was still ongoing and was far from being finished. As explained above, it was very misleading to claim that the patients benefited from the cell therapy.
6. Celixir’s conclusion
6.1 Celixir state: “After a subsequent review following Professor Murray’s concerns and FOI requests, the MHRA remain satisfied by our dossier and that the Brompton study should proceed.
It is difficult to believe that the MHRA would recommend that a company that has engaged in serious research misconduct by plagiarising and misrepresenting research data is fit to conduct a clinical trial. If true, this suggests that the MHRA is prioritising business interests over patient safety.
6.2 Celixir state:
“The independent experts from the Royal Brompton and the Royal Free have also reviewed the detailed information and found Professor Murray’s accusations to be baseless.”
Anonymous comments of this kind that are devoid of any specific criticisms are essentially meaningless.
It would be useful to know:
- the identities of the independent experts from the Royal Brompton and the Royal Free;
- which of my concerns they found baseless;
- their reasons for concluding my concerns were baseless.
7. Scientific Advice provided by the European Medicine’s Authority (EMA)
One of the documents relating to Celixir’s application for Clinical Trials Authorisation that was provided by the MHRA under FOIA, was the EMA’s Scientific Advice document. As outlined below, this document reveals some concerning issues.

6.1 The Scientific Advice document reveals that Celixir were requesting the EMA’s opinion on whether their cell product could be considered for conditional marketing authorisation on the strength of the data from the Greek trial.
It appears that Celixir provided misleading information to the EMA.
Specifically, Celixir informed the EMA that all 11 patients in the Greek trial were New York Heart Association (NYHA) Class IV, which is the most severe form of ischaemic heart disease. This information was incorrect. According to the 2016 paper that reported on the Greek trial, of the 11 patients, only 4 were NYHA Class IV; 2 were NYHA Class III; and 5 were NYHA Class II7.
Thankfully, the EMA did not endorse Celixir’s proposal. Their assessment is as follows:
“The proposal of the Applicant is not endorsed. The submitted data are not adequate to support a conditional marketing authorization (CMA) for several reasons. The data are limited to 11 patients, and are not controlled, precluding any robust conclusions on the reported endpoints. The use of historical data cannot substitute for an in-study control group. It is difficult to attribute the benefits to the injected cells without having a controlled arm of patients undergoing CABG only. The study was also single centre making it difficult to generalise the data….[..]. Further, the route of administration needs to be supported, preferably by nonclinical studies. The submitted results of the 11 patients are encouraging, showing a 100% MACE free survival at 28.4 months compared to a reported 8% survival in patients with NYHA IV [incorrect information provided by Celixir to EMA] with ICR post CABG (Marchenkoa et al, 2011; Gardia et al, 2013). However, long term controlled clinical outcome data are needed, especially in the field of stem cell research in cardiovascular disease where the results are very controversial and none of these cells are currently authorised. Regarding safety, the development of atrial fibrillation in 5/11 patients should be adequately addressed.”

6.2 It is also noted that Celixir appear to have misrepresented flow cytometry data obtained from peripheral blood-derived MSCs as bone marrow-derived iMPs, giving the impression that the iMPs are a different cell type to MSCs.
6.3 Celixir asked EMA the following question:
“Does the Agency [the EMA] agree that the available in-vitro and in-vivo data, together with published literature on iMPs, provide an appropriate non-clinical package, and that no additional non-clinical studies are warranted with this product?”
The EMA‘s response indicates that they did not agree:
“Taking into consideration that the cells represent a unique and distinct cell population in comparison to standard MSCs and that unique functionality is claimed, in vitro functional data with the product are needed to establish the (additional) mode(s) of action relevant for the therapeutic effect. When multiple modes of action are claimed functional data are needed to justify the selection of a potency assay that is relevant for the therapeutic effect. For this purpose an in vivo correlate preferably from patients is needed. If not feasible, data derived from a relevant animal model recapitulating at least some of the pathophysiological features of the condition to be treated (for example ischemia, incomplete vascularisation and contractile dysfunction) may be needed.”
The EMA make it clear that Celixir’s claims that the iMPs have unique functionality need to be backed up with data. It is therefore surprising that without these important data, the MHRA recommended that the Company should conduct a Phase II clinical trial at the Royal Brompton Hospital.
Update 27.09.2019
Today I received a message from Kadiya Qasem, assistant director of communications and public affairs at Royal Brompton. She referred to the statement by Celixir “The independent experts from the Royal Brompton and the Royal Free have also reviewed the detailed information and found Professor Murray’s accusations to be baseless”. The Royal Brompton statement was as follows:
“No review has been undertaken by Royal Brompton staff in relation to any accusations made by Professor Murray. The participation of Royal Brompton & Harefield NHS Foundation Trust as a participating site, in any research study, is subject to local review, to confirm arrangements for delivery of the study are in place. This is contingent on receiving all necessary regulatory approvals.”
Update 1.11.2019
MHRA Interim Chief Executive June Raine informed Murray on 21.10.2019 about that Celixir’s Phase 2b clinical trial at Brompton hospital must take place (original letter here):
“I can confirm that the trial was authorised in 27th September 2017, and this remains in place. The MHRA has conducted an internal review based on all information available to us, including the reports which you have provided, and currently has no grounds to take regulatory action to halt the trial.
We are unable to comment on accuracy of data in journal publications or patents, as these documents do not form part of the clinical trial authorisation application. However, your report and comments on these aspects have been reviewed and considered with regard to the need for any regulatory action.
The trial will be under close surveillance and our Inspections, Enforcement and Standards (IE&S) division is aware and fully engaged in aspects of the trial that fall within its remit. The manufacturing process is supported by the data provided and all required licences. This includes the starting material (which is not sourced from the UK), data for cell surface markers for more than one batch, and the manufacturers licence for the steps conducted in Greece. The switch from using peripheral blood to bone marrow is noted, however, this does not impact on the current trial for which the full manufacturing process has been established.
It should also be noted that the advice given by the European Medicines Agency in 2015 was from the perspective of data to support a marketing authorisation, when a full and complete data package is expected. This is different from the requirements for approval of an individual clinical trial, when data are still emerging and the product is still in development“.
Donate!
If you are interested to support my work, you can leave here a small tip of $5. Or several of small tips, just increase the amount as you like (2x=€10; 5x=€25). Your generous patronage of my journalism, however small it appears to you, will greatly help me with my legal costs.
€5.00



I obtained a 9 page work contract by Celixir. Employment was offered for one year with a possible maximum one year extension. The full document cannot be shared to protect the source, but here are some hair-raising passages from it:
“The Employer operates a policy of job flexibility and the Employer may, at its discretion, require you to perform additional or other duties, whether skilled or unskilled, not within the scope of your normal duties and may at its discretion amend your Job Description at any time.”
“Your normal place of work is our offices and labs at the Institute of Life Sciences in Swansea and Evans laboratory at Cardiff University. The Employer may require you however to work at such other locations on a temporary basis as the Employer may from time to time require. The Employer reserves the right to relocate you on reasonable notice to such other locations as the Employer may from time to time require.”
“Normal Working Hours
1.7 Your normal working hours are 42 hours per week to be worked at such times as the Employer reasonably requires.
1.8 You are required to work such additional hours as may be necessary or appropriate from time to time to enable you to carry out your duties properly. You shall not be entitled to receive any additional remuneration for work outside your normal hours.
1.9 The Employer reserves the right, if it reasonably requires, to increase, reduce and/or otherwise vary or alter your hours or times of work”
“Covenant Not to Compete
3.1 For a period of two (2) years after the Termination Date, you shall not, directly or through others, either acting alone, or as a stockholder, partner, associate, creditor, consultant, adviser, franchiser, franchisee, director, officer, owner, employee, representative or agent of any other person or entity, or in any other capacity, engage in or provide the same or similar services you provided to the Company or its affiliates during his service relationship to any entity or individual engaged in or preparing to engage in the Same or Similar Business (as defined below) in the Territory (as defined below); provided, however, the restriction contained in this paragraph 6 shall not prohibit you from owning not more than 1% of the outstanding stock of any class of any publicly traded corporation, so long as you do not actively participate in the operations of such corporation. For purposes of this Agreement:
– the term “Same or Similar Business” shall mean the Business as it applies to Regenerative Medicine including but not limited to mesenchymal stem cells and platelets or related mechanism of action or any other line of business in which the Company or its affiliates has invested time, money or other resources and with respect to which you have provided services to the Company or any of its affiliates at any time; and
– the term “Territory” shall mean any location in the world.”
“6.1 Subject always to the statutory minimum notice requirements, this Contract can be terminated by either party giving to the other not less than 2 weeks written notice within the 1 st year of employment and thereafter written notice of not less than 4 weeks is required.
6.2 The Employer reserves the right to pay salary in lieu of notice.
6.3 The Employer reserves the right to terminate your employment without notice or salary in lieu of notice in appropriate circumstances. Appropriate circumstances include, but are not limited to, situations of gross misconduct, gross incompetence and/or gross negligence.”
LikeLike
Hi Leonid
that “contract” sure sounds like slave labor and relocation at will! Does this “contract” even hold up under close legal scrutiny in the UK?
Oh, and that other shenanigans are pretty serious stuff too. If this is an example of the state of science and human subject protection in the UK then all bets are off.
The earlier, the better #BREXIT may come.
God save the Queen….
Cheers, oliver
LikeLike
It says here that a dose of iMPs will cost £72.5K (slide 13/17)…
https://ebrdg.com/alliancerm/investor.php/company/presentation/hash/8df27544cdeebb74f157f808cbafad88/c/43a7b0c010d11bc1245c5092ce72ad99
A vial of MSCs from AllCells costs a few hundred dollars.
Not a bad profit margin!
Strange that other companies haven’t thought of doing this.
Business model: buy some MSCs, transfer to a new vial with own brand name, call them something silly, make a big profit!
LikeLike
‘iMPs’ will get sent from Greece to Royal Free’s cell factory. This is run by Mark Lowdell who has his own stem cell company called LifePlus. https://lifeplusclinics.com/lifeplus-stem-cell-therapy
So the £72.5K must be to cover the cost of sending ‘iMPs’ from US to Greece; repackaging in Celixir-branded vials before sending from Greece to Royal Free; transport from Royal Free to Royal Brompton for injection into hearts.
LikeLike
But Mr Digbeards, do you not know who Mark Lowdell is? He is the man who makes stem cell tracheas & laringes for UCL and his colleague Martin Birchall! His speciality, aside of experimenting on children, is raising pigs from the dead: https://forbetterscience.com/2018/04/10/martin-birchalls-two-dead-pigs-to-prove-trachea-transplants/
LikeLike
What a tale! Bit of a contrast with this story that says a trachea he made saved the life of a youg boy and that he is repairing voice boxes of people with cancer. …https://www.gazette-news.co.uk/news/14975965.saving-lives-all-in-a-days-work-for-top-research-scientist/
LikeLike
Oh, but Prof Lowdell must suffer from some form of confusion. He never made a trachea for Ciaran Lynch in 2010, Macchiarini made that. Prof Lowdell did make a trachea for Shauna Davison in 2012. He might feel he saved her life, but the alternative reality she died 2 weeks after transplant, her mother is very angry at him and his colleagues.
https://forbetterscience.com/2017/04/20/ciarans-success-story/
https://www.telegraph.co.uk/education/2019/02/27/mother-girl-whose-death-part-alleged-cover-scientists-says-surgeons/
LikeLike
Hahaha
Ajan has corrected his title at Companies House. As of 7th Oct 2019 he is no longer Dr Reginald! That doesn’t excuse him from masquerading as a doctor though for all those years:
https://beta.companieshouse.gov.uk/company/07214755/filing-history
LikeLike
A strange-sounding UK company called ‘Mogrify’ that shares a Director with Celixir has just been awarded $16M to make ‘novel cell therapies’ https://techcrunch.com/2019/10/14/uk-biotech-startup-mogrify-injects-16m-to-get-novel-cell-therapies-to-market-soon/
Money comes from an investment institution called Ahren that includes none other than the boss of the UK Royal Society –Venki Ramakrishnan.
LikeLike
To be fair, Mogrify may be perfectly legit (same with the director) but now Ahren should do further due diligence due to the connection. I know that is what I would do!
LikeLike
There is a big clean-up operation going on. The Trepup site were Ajan was advertising himself as a medical doctor has been wiped.
LikeLike
This Trepup page about “Dr” Ajan Reginald?
https://web.archive.org/web/20190811230727/https://www.trepup.com/ajanreginald
http://archive.is/GsipQ
LikeLike
Hooray! I was afraid we might have lost him.
LikeLike
Regarding the company called ‘Mogrify’ that shares a Director with Celixir: CEO Dr Darrin Disley, claims to have been awarded as an Officer of the Order of the British Empire (OBE) in 2018. I did not find his name on the official list:
https://en.wikipedia.org/wiki/2018_Birthday_Honours
However, according to the University of Cambridge, he did:
https://www.ceb.cam.ac.uk/news/news-list/darrin-disley-awarded-obe
Is he really a doctor?
LikeLike
Disley’s there on page 51 of the 222-page list.
Click to access Queens_List_BD18.pdf
LikeLike
Does OBE really stand for that? I thought it meant ‘Odious Brown Effluent’.
Anyways, here some Glassdoor comments from employees of Horizon Discovery where this chap was CEO:
Avoid this company: “punching above their weight, lack the finances to delliver. Badly managed, and some managers are very unprofessional. A very unpleasant work environment. Many unhappy staff. Salary way off base, and they promise things they can’t deliver.”
From a Bioinformatician: “This is your warning. I was successful in my role. However, this was simply an awful place to work. The company has struggled with bioinformatics for a while. It was difficult place to get good work done. High turnover in the bioinformatics team. The skills that remained didn’t fill the gaps. Less awareness of business and how to position products than you’d think. Culture unlikely to change, even if certain individuals leave/have left.”
Avoid Horizon: crony management and abysmal payscale. “Low pay, forget about shares or just keeping up with the cost of life in Cambridge/London, especially if you have children. Scientists are not valued and when critical scientific projects get completed managers take most of the credit and barely mention who did the real work. Being close friends with senior management appears to be much more important than getting things done.” Advice to Management: “Record yourselves and pay close attention to what you are saying.”
LikeLike
Yes, I noticed it just now. I am sorry, Wiki is apparently not trustworthy…
LikeLike
Two search engines for finding doctors in Stratford Upon Avon bring up guess who?
Ajan Reginald!
https://www.inyourarea.co.uk/localservices/CV37%208SD/Doctors
https://directory.chesterstandard.co.uk/search/stratford-upon-avon/doctors
LikeLike
Wow he is still calling himself a doctor? That’s really outrageous how can this be legal…?
LikeLike
Pingback: Magic crystals and Nobel Science rules – For Better Science
Pingback: Murray vs Celixir: MHRA to sell British public for medical experiments? – For Better Science
Pingback: Steven Houser and the Temple of Fraud – For Better Science