Exciting news in Big Pharma, only from last year: in April 2018 the French multinational Sanofi recruited the former Roche executive John Reed as their new Head of Research & Development. As media reported, Reed previously left the Swiss pharma giant Roche “for personal reasons” in March 2018. The stint at Roche in Basel was relatively short, as Reed only switched there in 2013, having left his position as CEO of Sanford-Burnham Medical Research Institute in La Jolla, California. Prior to that, Reed had a career as academic, at University of California at San Diego (UCSD), he is also member of American Association for the Advancement of Science (AAAS).
Recently the data integrity sleuth Clare Francis pointed me towards some research papers on PubPeer, most of which are from Burnham Institute and signed by Reed as corresponding last author. I personally think Sanofi should distribute these Reed papers in all their research facilities worldwide to teach their employees how to science properly. Maybe complemented by the papers of Reed’s past collaborators Paul B Fisher and Paul Dent of Virginia Commonwealth University.
Reed’s scientific expertise lies in discovering new pharmacological targets for cancer research, a huge market in pharma business, and the following representative papers show how highly efficient cancer cures can be designed with the most minimal of technological investments. Quite some of them appeared at the Journal of Biological Chemistry (JBC) which is known to be needlessly mean to successful scientists (e.g., here and here). Let’s see how it works out for Dr Reed, Head of R&D at Sanofi, whose annual salary is probably higher that the entire budget of JBC‘s academic publisher, the American Society for Biochemistry and Molecular Biology.
Let’s start with an over 20 year old classic from Burnham.
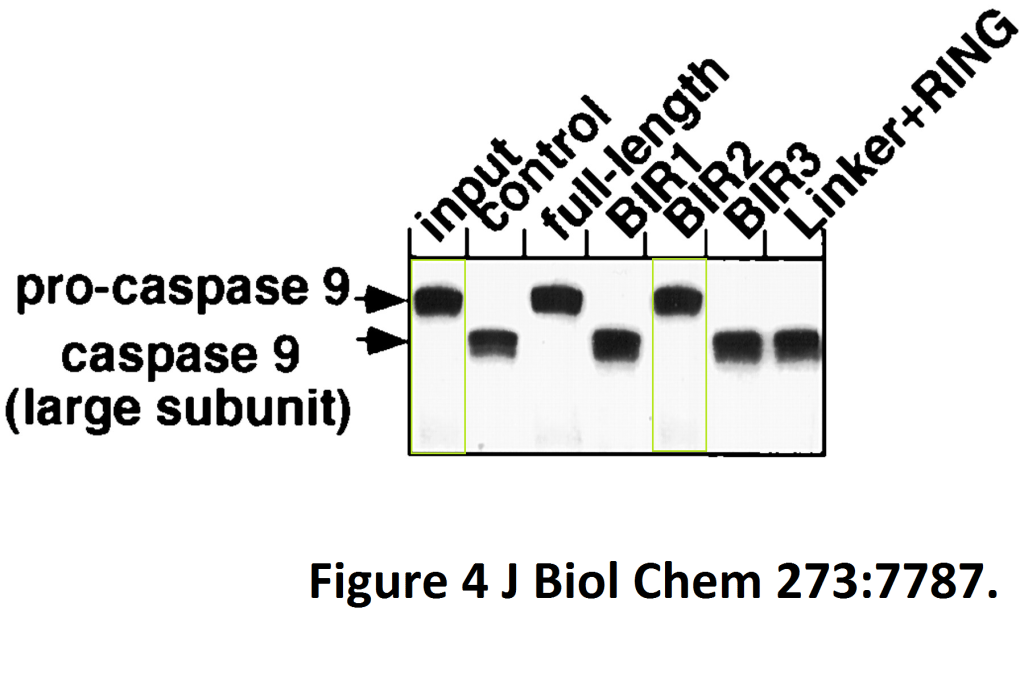
Ryosuke Takahashi, Quinn Deveraux, Ingo Tamm, Kate Welsh, Nuria Assa-Munt, Guy S. Salvesen, John C. Reed A single BIR domain of XIAP sufficient for inhibiting caspases The Journal of biological chemistry (1998) doi: 10.1074/jbc.273.14.7787
Here Reed and colleagues identified a novel caspase inhibitory domain which can become pharmacologically relevant when a cancerously-transformed gel lane divides while acquiring a metastatic invasive propensity in a gel-figure transgression assay.
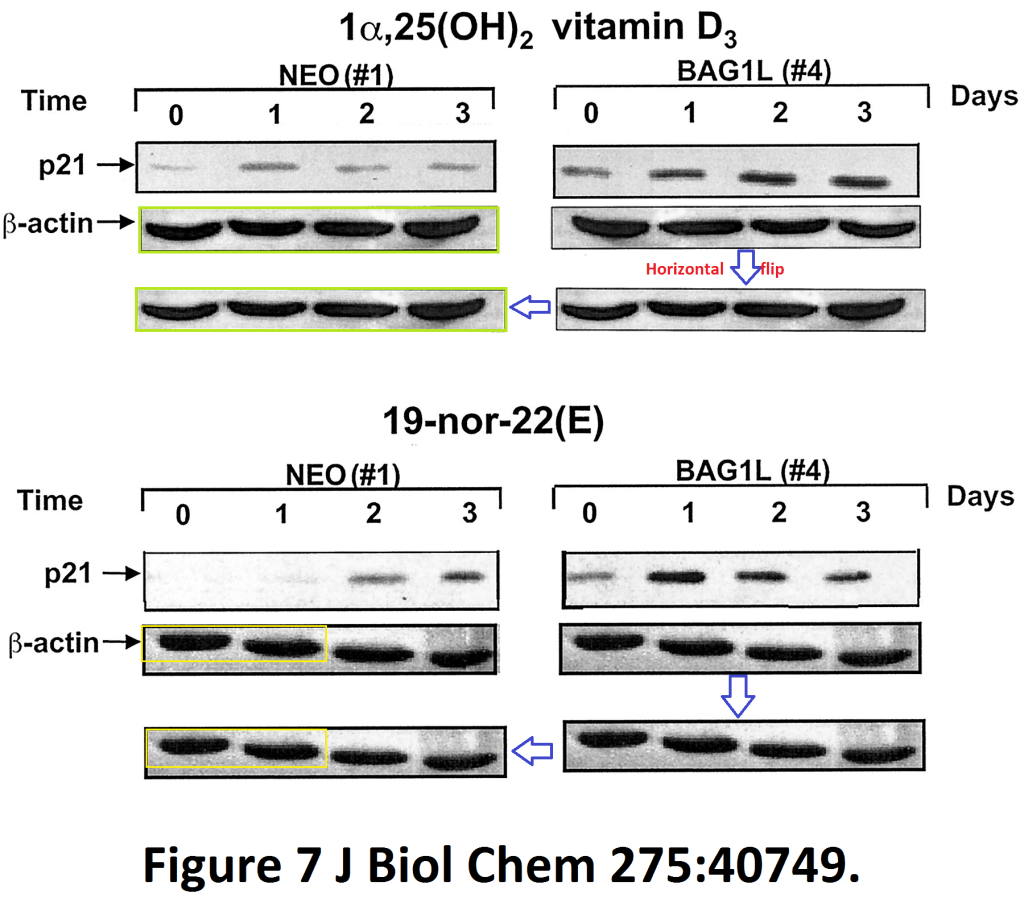
Meral Guzey, Shinichi Takayama, John C. Reed BAG1L enhances trans-activation function of the vitamin D receptor The Journal of biological chemistry (2000) doi: 10.1074/jbc.m004977200
The authors uncovered a mechanism with which Vitamin D receptor ligand BAG1L prevents proliferation of cancer cells by mirroring the metastatic invasion activity of dividing loading control bands:
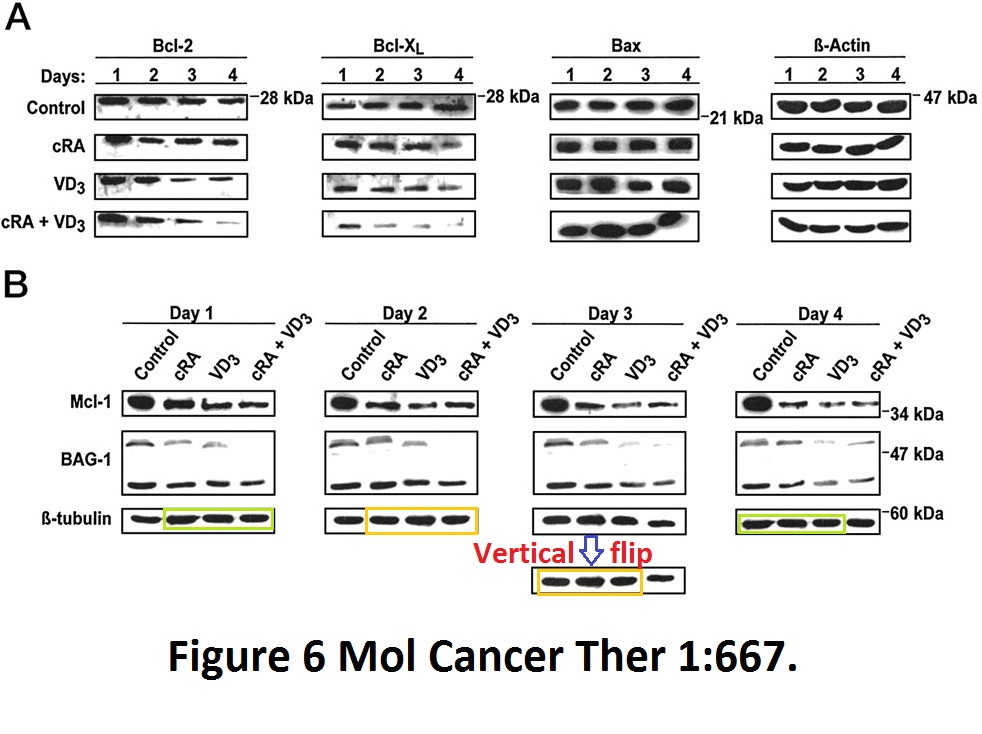
More from exactly same authors, where another Vitamin D receptor ligand, D3, proved itself as potent apoptosis inducer in cancer cells by inducing a rotational tetramer formation of the loading control:
Meral Guzey , Shinichi Kitada, John C Reed Apoptosis induction by 1alpha,25-dihydroxyvitamin D3 in prostate cancer Molecular cancer therapeutics (2002) Jul;1(9):667-77.
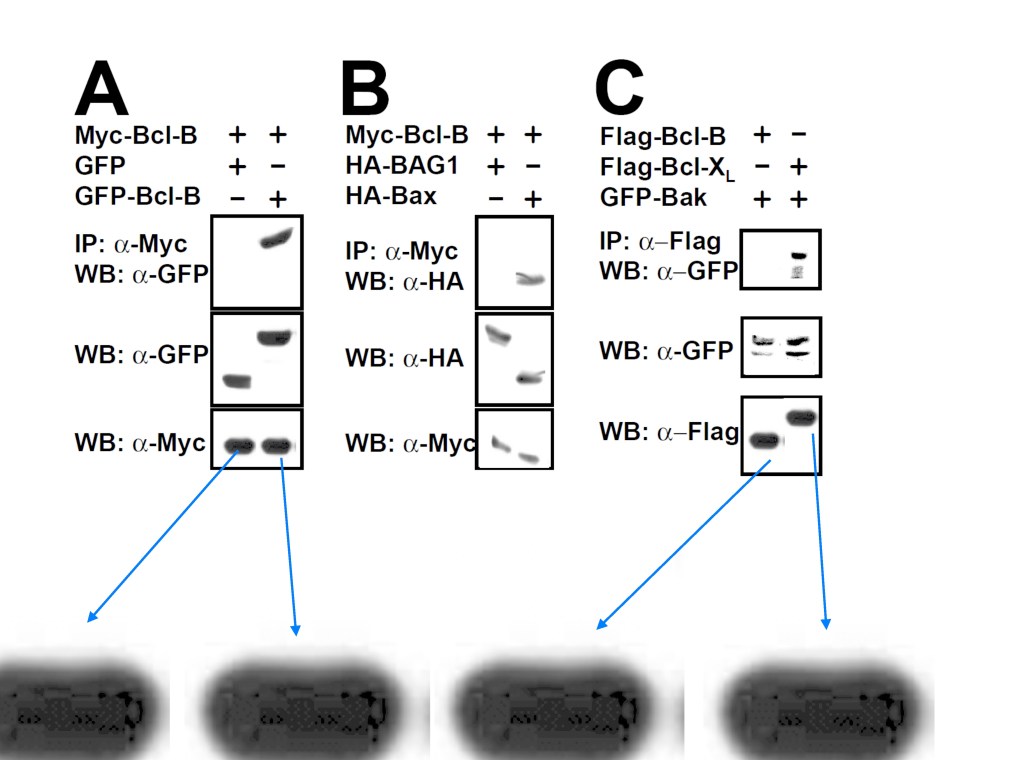
Ning Ke, Adam Godzik, John C. Reed Bcl-B, a novel Bcl-2 family member that differentially binds and regulates Bax and Bak The Journal of biological chemistry (2001) doi: 10.1074/jbc.c000871200
Here the authors discovered yet another novel protein, Bcl-B which proved a potent apoptosis suppressor, likely used by cancer cells undergoing clonal expansion due to oncogene-overexpressing gel bands.
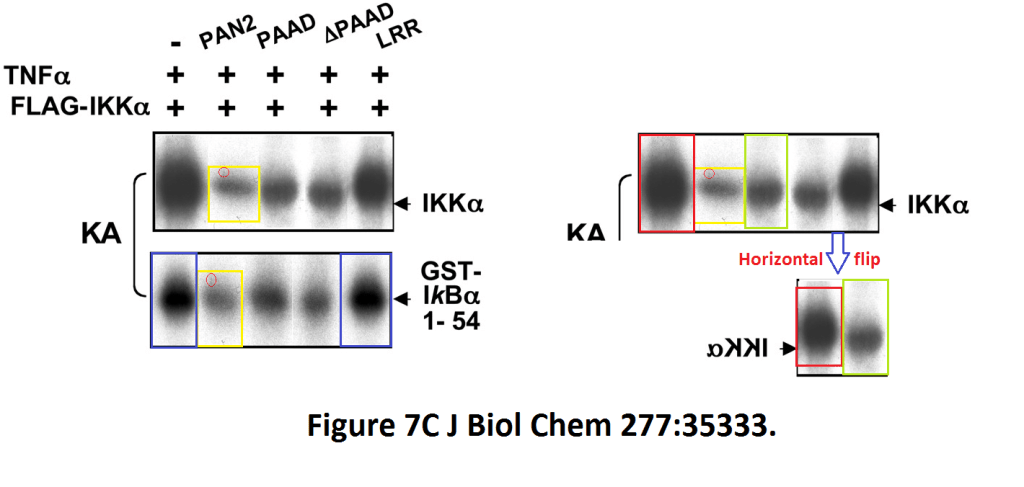
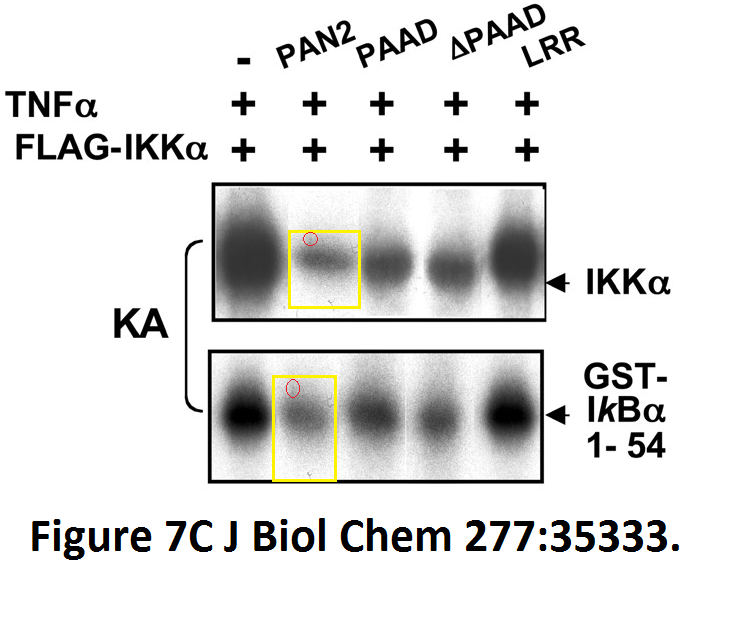
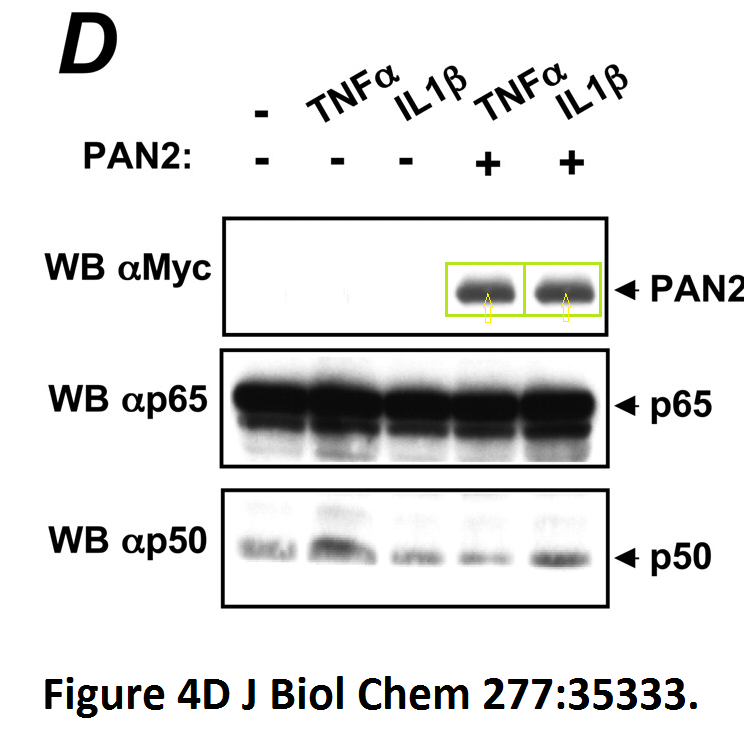
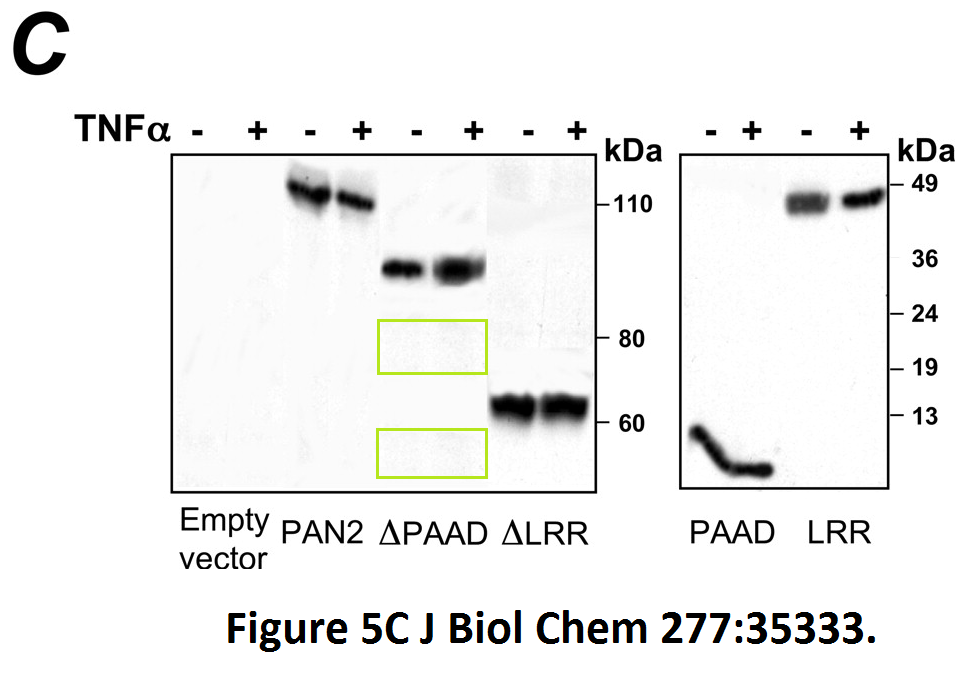
Loredana Fiorentino, Christian Stehlik, Vasco Oliveira, Maria Eugenia Ariza, Adam Godzik, John C. Reed A novel PAAD-containing protein that modulates NF-kappa B induction by cytokines tumor necrosis factor-alpha and interleukin-1beta The Journal of biological chemistry (2002) doi: 10.1074/jbc.m200446200
Here, a novel protein termed PAN2 was discovered which mediates inflammation by promoting high-rate mitotic division activity of bands and other usually non-dividing gel elements.
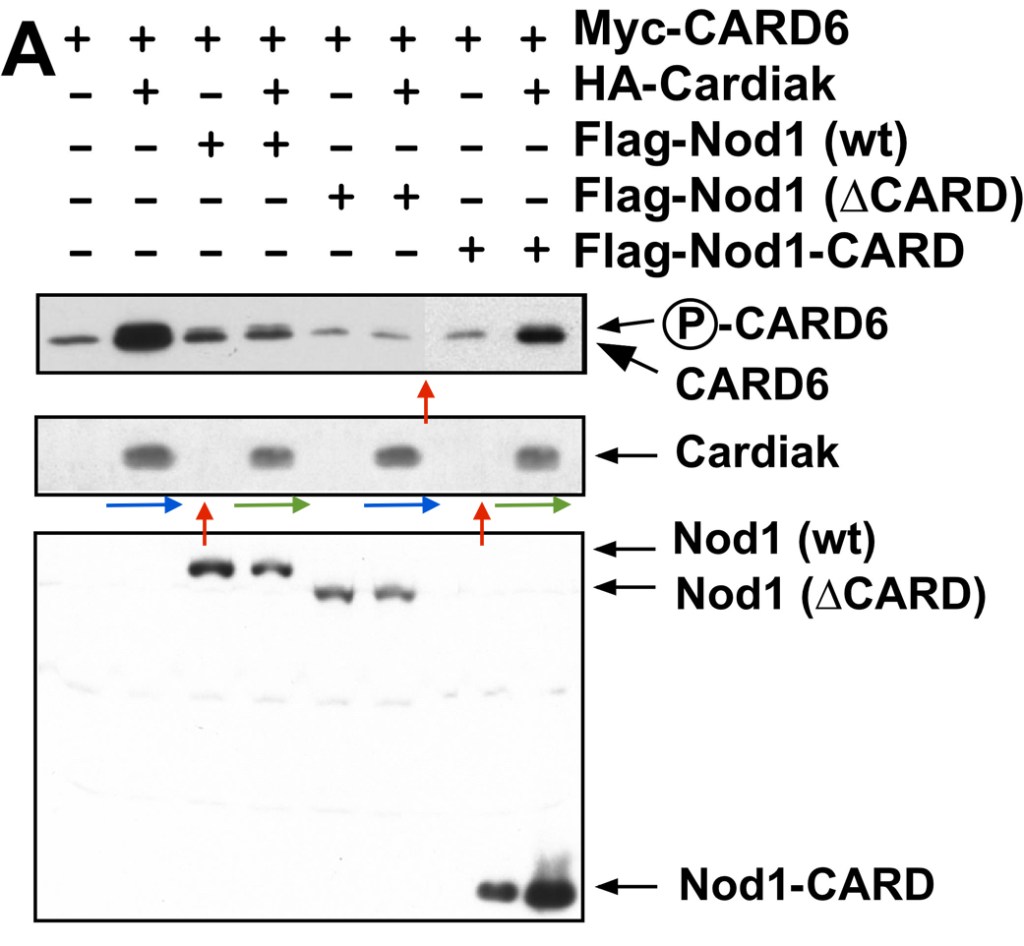
Christian Stehlik, Hideki Hayashi, Frederick Pio, Adam Godzik, John C. Reed CARD6 is a modulator of NF-kappa B activation by Nod1- and Cardiak-mediated pathways The Journal of biological chemistry (2003) doi: 10.1074/jbc.m300009200
Here, another immunomodulatory protein was discovered, CARD6. Its proliferative effects on gel bands are even more impressive, the signalling happens following the splice-event induced tertamerisation of Cardiak protein from two identical heterodimeric gel band subunits.
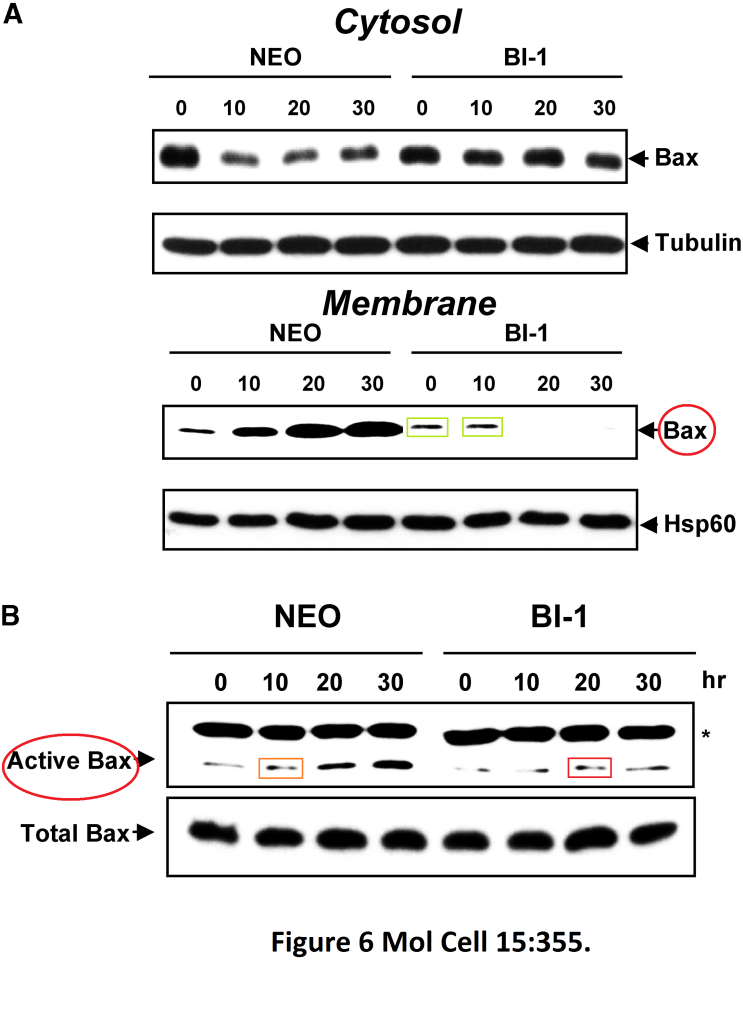
Han-Jung Chae, Hyung-Ryong Kim, Chunyan Xu, Beatrice Bailly-Maitre, Maryla Krajewska, Stan Krajewski, Steven Banares, Janice Cui, Murat Digicaylioglu, Ning Ke, Shinichi Kitada, Edward Monosov, Michael Thomas, Christina L Kress, Jeremy R Babendure, Roger Y Tsien, Stuart A Lipton, John C Reed BI-1 regulates an apoptosis pathway linked to endoplasmic reticulum stress Molecular cell (2004) doi: 10.1016/j.molcel.2004.06.038
Here, an inhibitor of the apoptotic protein Bax was studied, the authors discovered that BI-1 protein can prevent cell death by stimulating gel band mitotic activity in a research-integrity-refractory Molecular Cell.
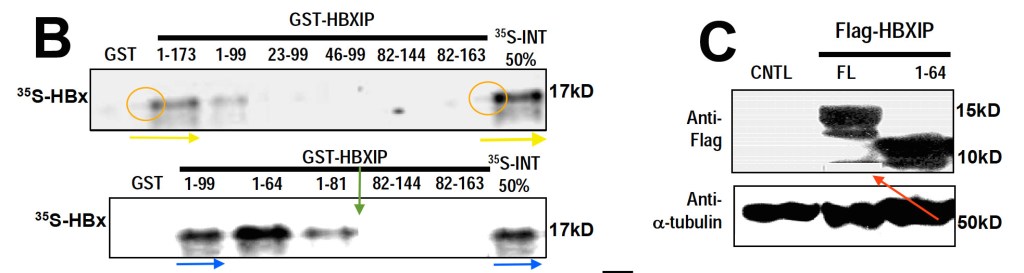
Yunfei Wen, Vladislav S. Golubkov, Alex Y. Strongin, Wei Jiang, John C. Reed Interaction of hepatitis B viral oncoprotein with cellular target HBXIP dysregulates centrosome dynamics and mitotic spindle formation The Journal of biological chemistry (2008) doi: 10.1074/jbc.m708419200
Here Dr Reed probably decided to follow-up in the mysterious mitotic activities of gel bands in his previous papers and found that these were caused by a chronic Hepatitis B infection of the western blot apparatus. The virus apparently can even cause band bleaching during oncogenic band-self-replication.
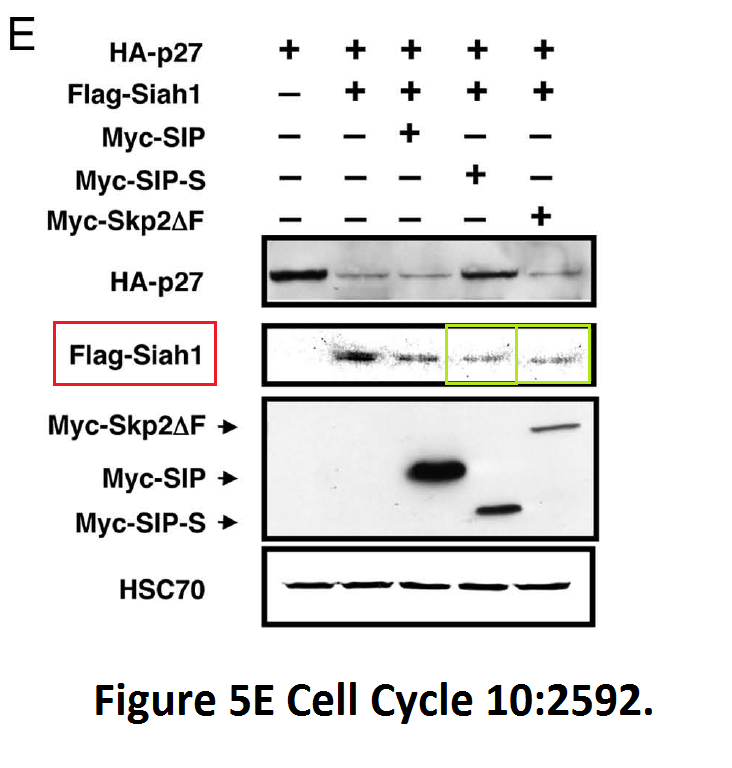
Obviously Burnham institute was tremendously successful in its drug discoveries under Reed’s leadership. This is a more recent paper it published, with the director as penultimate author:
Yoshito Nagano, Toru Fukushima, Kazuo Okemoto, Keiichiro Tanaka, David D.L. Bowtell, Ze’ev Ronai, John C. Reed, Shu-ichi Matsuzawa Siah1/SIP regulates p27(kip1) stability and cell migration under metabolic stress Cell cycle (2011) doi: 10.4161/cc.10.15.16912
The authors studied here the effect of glucose starvation on research integrity, and found that the effect is devastating, while mediated by an abnormally formed Siah1-homodimer.
In 2015, Dr Reed was already in charge of an oncology branch at Roche. His Burnham lab published this, while Dr Reed provided his new Roche affiliation:
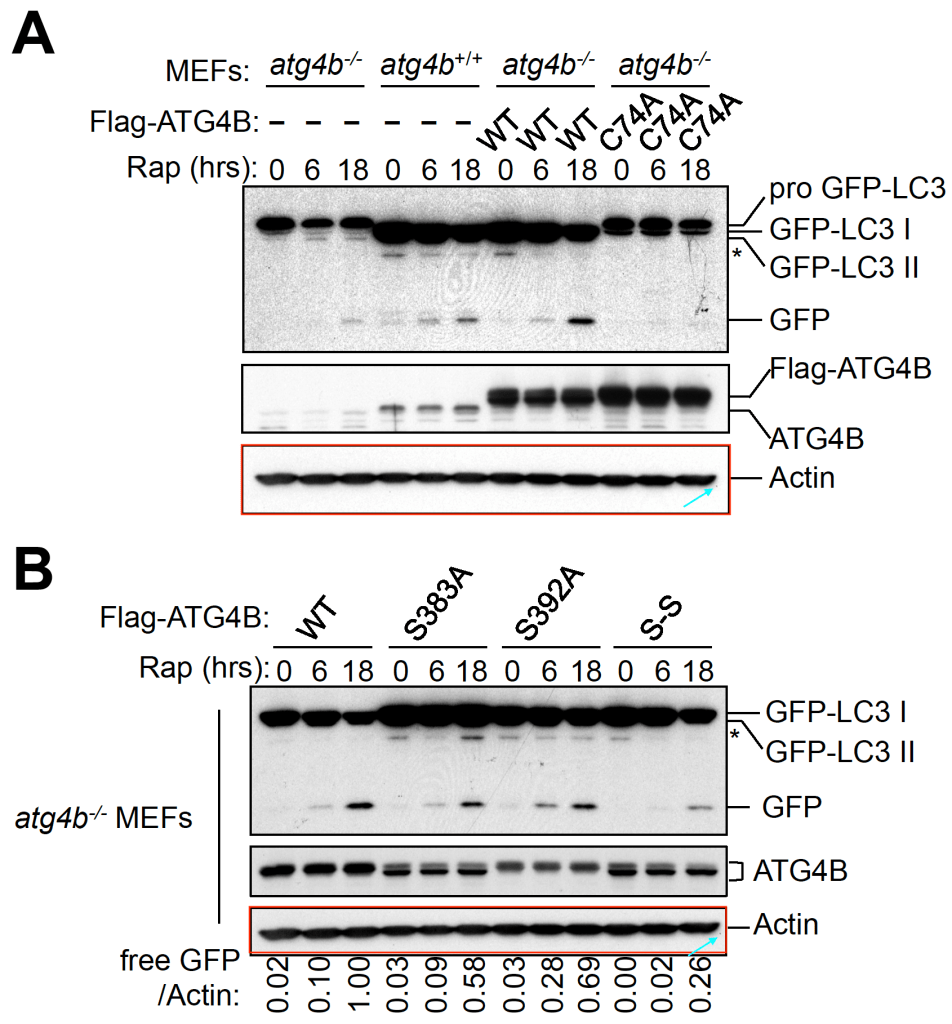
Zhifen Yang, Rachel P. Wilkie-Grantham, Teruki Yanagi, Chih-Wen Shu, Shu-Ichi Matsuzawa, John C. Reed ATG4B (Autophagin-1) phosphorylation modulates autophagy The Journal of biological chemistry (2015) doi: 10.1074/jbc.m115.658088
Here it turned out that phosphorylation of the autophagy-relevant protein ATG4B somehow leads to one loading control being eaten. It is then replaced by a de-novo synthesised clonal actin element undergoing lightness-induced rotational cropping activity.
The following 17-year-old collaborative study of Reed’s Burnham Institute with the University of Chicago provided some mechanistic insights into the gel image complex formation process induced by rigorous drug discovery research.
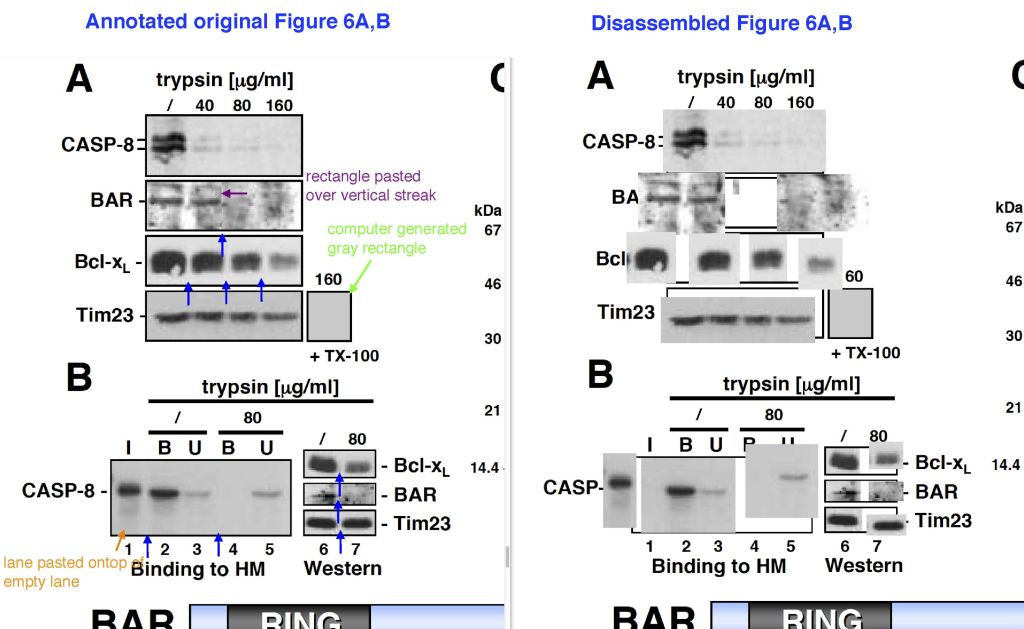
Alexander H. Stegh, Bryan C. Barnhart, Jorg Volkland, Alicia Algeciras-Schimnich, Ning Ke, John C. Reed, Marcus E. Peter Inactivation of caspase-8 on mitochondria of Bcl-xL-expressing MCF7-Fas cells: role for the bifunctional apoptosis regulator protein The Journal of biological chemistry (2002) doi: 10.1074/jbc.m108947200
The journal JBC namely saves images in pdf as they were submitted, so if an image is a composite one, it can be disassembled into the individual images it was made of. Some gels proved to consist of several malfunctional subunits, assembled by a hitherto unknown novel mechanism. Reed’s Burnham Institute apparently decided more research was needed, see above and here on PubPeer, a total of 40 papers from between 1998 and 2015.
I think Sanofi made a genius choice with Dr Reed as their Head of R&D, every patient should flip from excitement and at least double or even four quadruplicate their trust into Sanofi’s research and novel therapies now. And as the high achiever Reed himself teaches, successful pharma research depends on sacking people who do not perform:
Update 16.09.2021
Over two years have passed, and guess how many of these reed papers were retracted? Zero. Apparently, not even a single correction. With some more recent finds, Reed’s PubPeer record now stands at whooping 46 papers, and nobody dares to touch them.

 , Barbara Froesch , John C Reed , Marie-Paule Merville , Vincent Bours Inhibition of the NF-kappa B transcription factor increases Bax expression in cancer cell lines Oncogene (2001) doi: 10.1038/sj.onc.1204343
, Barbara Froesch , John C Reed , Marie-Paule Merville , Vincent Bours Inhibition of the NF-kappa B transcription factor increases Bax expression in cancer cell lines Oncogene (2001) doi: 10.1038/sj.onc.1204343 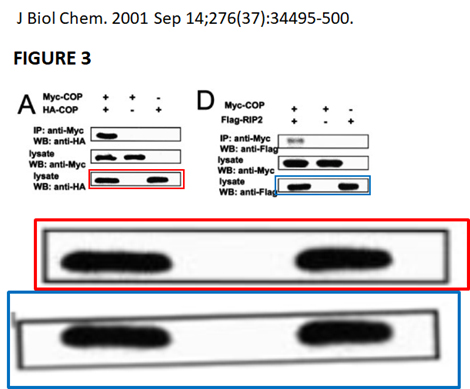
But a reader told me something interesting about Reed’s past. He once had to issue a correction to declare his conflicts of interests:
“In “Authorship” on page 3327, the conflict-of-interest disclosure should have included the following: “Dr Reed is a consultant to and shareholder in Apoptos, Inc, Coronado Biosciences, Inc, and ISIS Pharmaceuticals, Inc, as well as the sole inventor of Genasense™ (oblimersen sodium).“
Reed invented Genasense while mentored by none other but Carlo Croce, and licenced the drug to the company Genta, on whose board Reed used to sit and into which his current employer Sanofi invested almost $500, all of which went up in smoke. There was an article about this Genasense scam, published by Jim Silverman in 2012 on now defunct The Street, but here a backup. It kind of lets one wonder if Sanofi’s long-term plan is to destroy their company from inside. I quote the article in full:
Genta: 1998-2012
BOSTON (TheStreet) — On August 2, Genta (GNTAQ) filed for Chapter 7 bankruptcy liquidation, thereby ending 24 years of repeated clinical failures, multiple FDA rejections, countless toxic financings and epically piggish executive behavior.
Before the Genta corpse rots into distant memory, I thought it might be helpful to document the company’s colorful and painful history. The Genta story is a warning to biotech investors showing how chronic clinical failure can lead to soul-crushing shareholder dilution. [Cell Therapeutics (CTIC) shareholders — pay special attention.]
Old-timers may recall that Genta was founded with the mission to develop generic versions of hard-to-manufacture drugs. The company’s first target in the late 1990s was the calcium channel blocker Procardia XL, which at the time was among the world’s biggest selling branded drugs. Generic drug makers were keen to develop their own versions of Procardia. Genta was in the hunt too, and thought it had an advantage because of a proprietary drug delivery technology licensed from a European company called Jagotec. Unfortunately, Genta’s efforts flopped and other generic drug makers copied Procardia first. None of Genta’s generic drug projects ever made it to market and the entire program was eventually scrapped.
Enter Genasense, an “antisense” drug designed to block a key protein involved in various cancers, including certain leukemias and skin cancers. In the early 2000s, antisense was big news, the “flavor of the day” biotech technology, much the way RNA interference rose to prominence more recently. Genta promoted Genasense relentlessly despite murky evidence of efficacy. All that hype paid off in 2002 when Aventis (now Sanofi (SNY)) agreed to partner with Genta on Genasense’s development.
It’s hard to believe now, but Genta traded on the Nasdaq back then and was considered a real biotech company — albeit one steeped in controversy. The Aventis deal — $480 million in up-front cash payments and development milestones — was a high water mark for Genta and Genasense. It went downhill from there for the company and its shareholders.
This where the Genta story becomes more familiar. Genasense’s phase III study in melanoma failed, although Genta claimed the data were positive. The company sought FDA approval but the drug was rejected. Genta tried for FDA approval again, this time with lackluster data from a leukemia clinical trial. Again, FDA kicked Genasense to the curb. Genta decided to give melanoma another shot but a do-over clinical trial failed. Finally, in 2011, Genasense was scrapped.
It costs a lot of money to run a drug development program with this level of ineptitude. Of course, all this money was essentially thrown away, leaving shareholders holding stock diluted into oblivion through an endless series of financing and reverse stock splits. This is where the Genta story boggles the mind.
Take a look at Genta’s catalog of reverse stock splits:
1997: 1 for 10
2007: 1 for 6
2009: 1 for 50
2010: 1 for 100
2011: 1 for 50
2011/2012: FINRA denied multiple Genta requests for yet another reverse split, evidently saying enough is enough.
July 2012: Genta shareholders approve a “reincorporation” of the company from Delaware to California, merging Genta Delaware into Genta California, effectively trying to game the system so they could institute an up to 1-for 25,000 share split.
Multiplied together, Genta pushed through an astounding 1-for-15 million reverse stock split, beginning with their initial split in 1997 and ending in 2011.
A 1-for-15 million reverse stock split is hard to grasp but think about this way. If you had owned 15 million shares of Genta in 1997 and never sold a single share, today you would own 1 Genta share.
In 1997, Genta had slightly more than 40 million shares outstanding so that means there are just two of these Genta common shares remaining. Their value today: $0.0006.
Okay, here’s where Genta gets downright freaky. Let’s assume Genta never instituted any reverse stock splits. How many outstanding shares would the company have on its balance sheet today?
The answer: Approximately 100 quadrillion shares outstanding!
This is what 100 quadrillion looks like numerically:
100,000,000,000,000,000
Mind you, 100 quadrillion shares only represents Genta’s common stock (15 million in splits multiplied by roughly 6-7 billion shares currently outstanding.
The fully diluted share count, sans reverse stock splits, would be 1 quintillion!
This is what 1 quintillion looks like numerically:
1,000,000,000,000,000,000
A history of Genta is not complete without mention of Dr. Ray Warrell, chairman and CEO since 1999. In 13 years, Warrell manned Genta’s helm through all but one of the reverse stock splits, the failed Genasense program, $1 billion in accumulated losses and an almost 100% plunge in the company’s stock price. At Genta’s end, Warrell was still chairman and CEO, surely a record for futility and resilience.
And at his side, through it all, stood Genta’s chief medical officer Loretta Itri, also known as Warrell’s wife. Surely, the Warrell/Itri combo must be near the top of any biotech Power Couple list.
In reward for their ignominious track record — and to “incentivize and retain” the duo — Genta’s board of directors, just six weeks prior the filing bankruptcy, awarded Warrell and Itri a combined 1.45 billion shares of Genta restricted stock.
Alas, as they say, all good things must come to and end. On August 2, biotech flags around the world were lowered to half-staff to recognize Genta’s filing of Chapter 7 bankruptcy liquidation.
That Genta was able to survive for 24 years, burn through $1.2 billion dollars of investors money, finance themselves through deeply discounted converts for over two decades, with a single CEO for its final 13 years, even after losing 100% of shareholder value, is truly a remarkable, perhaps never-to-be-replicated story.
R.I.P. Genta.
Silverman has no position in Genta — thankfully.
Donate!
If you are interested to support my work, you can leave here a small tip of $5. Or several of small tips, just increase the amount as you like (2x=€10; 5x=€25). Your generous patronage of my journalism, however small it appears to you, will greatly help me with my legal costs.
€5.00


I remember many years ago visiting Dr.Reed’s lab at Burnham institute seeking for a an opportunity to do my PhD there….by the time Dr.Reed was already known as “the paper machine”, 20 papers per year, 40 postdocs in the lab…he doesn’t have time to neither to make corrections or even read all these papers!!
LikeLike
….and all of the those post-doc’s probably have great jobs in industry, even if the work they did was unreproducible. You have a stark choice in scientific research: Be perfectly honest and likely “fail” (meaning, be not-so-well paid and be held in scorn by family members and society (at least in America)), or be dishonest and “succeed” (be a liar who makes tons of money and held with respect by his contemporaries)…..
LikeLike
And I’m wondering why you guys, that complain as “honest scientists”, do not have any remarkable work in terms of academic position, publication, grant, etc. For example, you, Ana Pedro, which I guess this is your publication record here: https://scholar.google.com/citations?user=-8DTkV8AAAAJ&hl=pt-PT . If this is your publication record, I would say you are a loser and you did not have any chance to get into John Reed lab. In fact, just one of John Reed publications has citations more than all of your publication record (if that is really you!) : https://gut.bmj.com/content/64/1/66.short . So, besides accusing people here and get a gesture of being under represented by the “family members and society”, roll up your sleeves and do some research instead of throwing nonsense…
LikeLike
Mike, June 16, 2019:
“It’s not that easy…. sometimes faking data is the only way to survive for some people.”
So I guess you are saying roll up your sleeves and get to work on that photoshop, and then you have a right to brag about how great you are, and you are not a “loser”. So, dignity and self-worth depends on status and not honesty. Maybe you should apply in the Trump administration…
LikeLike
I don’t have anything against Dr.Reed who I didn’t get to know well. By the contrary, I was extremely well received at the Burnham Institute by the time. What I commented previously was the general impression you could take at the time about Dr.Reed. Also, Dr.Reed accepted me for the PhD in his lab, I didn’t go to his lab, because I was strongly advised not to do it by trustful anonymous people and because it was hard to find an academic at an European university who would mentor such a PhD at an American Institute. I ended up doing my PhD with not so famous mentors, my PhD was a cross, I didn’t publish my PhD work but I found academics dedicated to me really willing to transmit me good and honest research skills and not treating me as such more one PhD student in a big lab where the famous lab head is so busy and doesn’t have time just for more one PhD student
LikeLike
Well, he can sort of be directly absolved of the fraud if the lab was so big that it’s impossible to supervise for these things. If even 10% of the postdocs are dishonest, then that means 4 people with four papers a year (since they would be more productive). It can add up even if 90% of the postdocs are honest scientists.
However, this means that he wasn’t an effective leader of that lab with 40 postdocs since convincing people not to cheat is one of the most important managerial things. I imagine it would matter a whole lot more at a pharma company if 10% of the researchers there are fudging data. Based on his academic managerial record, even if Dr. Reed is a person of integrity, his Pharma company should be worried about his job in charge of research with them.
LikeLike
I remember there was an environment of very big competion between the postdocs of Reed lab. There wasn’t the feeling they were part of a big team but instead it was like small independent groups co-existed within the lab. I think within a company or institute or university which intends to develop any type of biomedical research groups of scientists with different backgrounds and expertise must be involved and assigned specific projects. Moreover, I keep believing in this case the original data should be available in a database similarity to the human genome project and the review of the results must be public.
LikeLike
@blatnoi you seem to not know the most basic facts about being lab head/PI. YOU are 100% SCIENTIFICALLY, LEGALLY and MORALLY responsible for what your group publish. If you cannot accept this you should not be PI. If you can not deliver it you are a shit PI. If you claim you can do this but afterwards is proven wrong you should RESIGN.
In this case its highly doubtful he did not know precisely what was going on.
LikeLike
This argues that PI’s should be limited in the number of post docs they oversee, Ii they cant effectively oversee, say, more than five (I work for an MD/PhD and I am his only post doc, and he doesnt really make any attempt to oversee me!). One way that can be controlled is by limiting NIH funding two 2 grants, and that should be extended to all federal funding in the US.
Another way to solve the problem is to pay post-docs a wage with decent job security (ie, not grant funded) so that they can have a decent middle class lifestyle, so they are less tempted to cheat. This might help are fun poster “Mike”, for example. But that goes against the belief that the US is a perfect meritocracy that is held by people in authority in science, and that only the “best” should be rewarded with a good job. The rest of you can just become the precariat. Also, the fact is the more hands in a lab, the more work is done and the more likely the PI will get publications, so it really doesnt makem a lpotn of sense for the PI to pay anybody well.
I think science was probably the most ethical was when only the independently wealthy could do it. Now, its being corrupted by individuals who cannot accept a lower middle class lifestyle of a honest scientist.
LikeLike
To Rex Rictor:
Where these basic facts about being lab head/PI are written? Even fathers and mothers that bring a person to this world are not “100% SCIENTIFICALLY, LEGALLY and MORALLY” responsible for their child actions, so why you demand people to take responsibility of works for someone as a post-doc that barely know them for a year maybe…
LikeLike
To NMH:
Who is the “best” in your meritocratic world?! You? Ana Pedro? Leonid Schneider? Who? How do you measure for being “best” in academia? By publishing? So why you accuse people that publish too much as garbage printers? That’s the key point to be “best” in your “meritocratic world”? Only “best” people should have decent pay and could hang in middle class way of life? Other people should not have decent pay and be in always stress of money and maybe die of course in your meritocratic world yes? Cause they’re not human due to the fact that they’re not “best” in your meritocratic world… The only hope is that nobody in academia takes you guys serious.
LikeLike
“@blatnoi you seem to not know the most basic facts about being lab head/PI. YOU are 100% SCIENTIFICALLY, LEGALLY and MORALLY responsible for what your group publish.”
Yeah, very idealistic. Great. But all it shows is that you either don’t know the basic facts about being the lab head/PI, or you’re a romantic like Schneider. Unlike the navy where the captain is fired for every mistake, it makes a huge difference if the intent to cheat, or even pressure others to cheat, can be demonstrated. And it’s not even a question of whether your sentiment is the actual ‘law of science’, because first of all it isn’t, and second these laws differ by country and institution. You obviously don’t read this blog that much, hence you don’t understand why someone like Latchman would try so hard to prove that he had no idea all this cheating was going on even though he admitted cheating was going on in his lab. Society will forgive a criminal who commits the crime without intent much more readily. Same for cheating in science no matter who is in charge of whom or who has stars next to their name and is ‘100% responsible’ for all content. We all put off small tasks and then wing it, or get tickets for speeding after all. This person was probably just trusting his subordinates too much… Why do you think you get much shorter terms for second degree as opposed to first degree?
LikeLike
“I think science was probably the most ethical was when only the independently wealthy could do it. Now, its being corrupted by individuals who cannot accept a lower middle class lifestyle of a honest scientist.”
It’s was a profession in many European countries in the 20th century. Where you probably have one student, who then works with you for 15 years as they become ‘postdoc’ and then ‘junior researcher’. A second student during that time period might leave to go work for industry. Of course it meant entry was highly restricted and people actually failed out, and the salaries were not too high anyways. Still, it allowed for ambition to some degree, but not on the American lab scale. If a person become institute director, they could put their name on every paper and become a ‘superstar’ that way, but they were not really in charge of the research in the junior groups.
LikeLike
What I am trying to say, my very delightful if somewhat sneaky friend, is that, IMO, everybody in the system of academic science is equally, or almost equally, important to the system of generating accurate, reproducible data. For this reason, the data generators should have as good of a job as the managers of the data generators. In my system, there is NO best. At least, in the sense where the “best” are paid good salaries with perfect job security.
But the way it is, these “managers” (a misnomer, because most make no attempt to manage) judge who is “best” and is deserving to be of their class, and who is not. The “best” get the rewards (good job), the non-best join the precariat. This is going to create a lot of fraud and anger.
For many years I really didn’t understand why there was a cultural revolution in China in the 1960’s, where Mao, in part, ordered the state execution of university intellectuals. This is an absolutely horrific thought, but if Mao was bossed around by overpaid-snotty-cannot-adequately-manage academics, maybe he just couldn’t control his anger.
LikeLike
Business is business, not Mother Theresa. Businessmen admire sharp practice. Leonid’s article has done John Reed a power of good.
LikeLike
John Reed left Sanofi yesterday to join Johnson & Johnson today.
https://www.businesswire.com/news/home/20230213005599/en/Johnson-Johnson-Appoints-Dr.-John-Reed-as-Executive-Vice-President-Pharmaceuticals-RD
LikeLike
Funnily enough, I think the stock price will go up. Isn’t that the way of the world?
LikeLike
A single BIR domain of XIAP sufficient for inhibiting caspases The Journal of biological chemistry (1998) doi: 10.1074/jbc.273.14.7787
Penultimate author, Guy S Salvesen, has 2013 Expression of Concern for a 1997 paper.
https://retractionwatch.com/2013/10/31/he-said-she-said-journal-of-neuroscience-expresses-concern-but-doesnt-pursue-investigation/
LikeLike
Guy S Salvesen also has a 2014 correction for a 2010 paper.
https://pubpeer.com/publications/10E0E38BD09A8787FC357AECE85B69
LikeLike
John C Reed has a 2004 Blood retraction in Blood.
https://pubpeer.com/publications/6331B245F4A8397C228F5C87A77987
Penultimate author of 2004 Blood paper, Thomas J Kipps, has these papers:-
https://pubpeer.com/publications/847996604CD73AE0DBF3B1E277FEEF
https://pubpeer.com/publications/7A0B3F6BA933BEEB6394FC9AE3BA5A
https://pubpeer.com/publications/FF222910D7DF9AE7F180ADFE1A102C
https://pubpeer.com/publications/363559ACFDAB5BD8D9B5F12F5FA56B
https://pubpeer.com/publications/D567B5F3B86B42900C486918ECC612
LikeLike
It’s reassuring when others find more problematic data.
J Biol Chem. 2002 Sep 20;277(38):35333-40. Epub 2002 Jul 1.
A novel PAAD-containing protein that modulates NF-kappa B induction by cytokines tumor necrosis factor-alpha and interleukin-1beta.
Fiorentino L1, Stehlik C, Oliveira V, Ariza ME, Godzik A, Reed JC.
Author information
1
Burnham Institute, La Jolla, California 92037, USA.
Figure 7C.
LikeLike
J Immunol. 2008 Apr 1;180(7):5045-56.
Salmonella secreted factor L deubiquitinase of Salmonella typhimurium inhibits NF-kappaB, suppresses IkappaBalpha ubiquitination and modulates innate immune responses.
Le Negrate G1, Faustin B, Welsh K, Loeffler M, Krajewska M, Hasegawa P, Mukherjee S, Orth K, Krajewski S, Godzik A, Guiney DG, Reed JC.
Author information
1
Burnham Institute for Medical Research, La Jolla, CA 92037.
Figure 8. More similar than you would expect.
LikeLike
Mike apparenly measures success in terms of money. Scientifically John Reed is a looser, which has been revealed through the long list of data manipulations and fabrications posted at Pubpeer. Based on this record I do not trust anything published by him. The pharmaceutical industry do not care about scientific accuracy, as long as they can maintain their profits. In that way Mr Reed fits well.
LikeLike
Pingback: Opera Buffa di Guido Kroemer a La Scala – For Better Science
Very interesting points.
I can make a connection with Didier Raoult here. https://forbetterscience.com/2020/03/26/chloroquine-genius-didier-raoult-to-save-the-world-from-covid-19/
I will be a bit approximative for generalization, don’t take everything for granted but it is for a global picture.
French public research system is based on 2 big institutes, INSERM (medically applied) and CNRS (fundamental science).
This system worked back in the 20th century as a publily funded research system with independant scientific decisions.
But as french research system was on the decline, a switch was decided towards a “project” logic, with political orientations, public funding being distributed by mixed authorities (political / scientifical). At the same time, for higher fundings, foundations were developping.
Under president Sarkozy (2007-2012), a new step was decided towards less independant research. Big Institutes were funded with public money in order to start institutes with a “foundation” status. Which means, an institute can get directly funded by private companies, which was not the case before. These institutes were called “IHU”, and politically active scientists could get positions this way.
That is how pr. Raoult got funded 32 Million € to create his Marseille institute. Source in french here :
Click to access rapport-IGAS-2015-008-IHU-Marseille_496367.pdf
Point number 45 in the document.
This Institute then started very strong collaborations with Sanofi.
Although no mention of Sanofi in his 142 page C.V. http://www.infectiopolesud.com/IMG/pdf/C-V-Didier_RAOULT-_President_Infectiopole_Sud.pdf
He led Infectiopole Sud with Sanofi participation : http://www.infectiopolesud.com/spip.php?article25
His funded institute logic made him also work in a larger group including his institute with again high Sanofi funding :
Click to access EUROBIOMED.pdf
and always inviting Sanofi to his big conferences :
https://www.eurobiomed.org/fr/evenements/evenement/key-advances-on-immunotherapy-and-cellular-therapy-against-cancer-and-inflammatory-diseases/
We can for instance quote the nivachick project on page 4 :
http://docplayer.fr/46587650-Ce-document-fait-le-point-de-la-situation-du-pole-de-competitivite-orpheme-ainsi-que-les-objectifs-pour-la-fin-de-l-annee-2007-et-l-annee-2008.html
I was long wondering why a human being could, in a public health crisis like COVID19, fake results in such a bad manner : https://forbetterscience.com/2020/03/26/chloroquine-genius-didier-raoult-to-save-the-world-from-covid-19/
What motivations can lead someone to cause people compulsively buy PLAQUENIL (c) SANOFI ?

How come politicians in France decide to give public money to save a closing SANOFI related company called FAMAR, owned by Kohlberg Kravis Roberts & Co (administrated by french Xavier Niel, very influent manager owning phone companies and media), and working for SANOFI in producing chloroquine ? Don’t they have enough money to support that company in such a crisis time ?
Sources :
KKR owns FAMAR and FAMAR is related to Sanofi: https://www.miroirsocial.com/participatif/famar-lyon-en-redressement-judiciaire-les-consequences-du-desengagement-de-lindustrie
KKR is led by Xavier Niel : https://www.lesechos.fr/2018/03/xavier-niel-rejoint-le-prestigieux-fonds-kkr-985684
FAMAR is closing : https://www.usinenouvelle.com/article/en-redressement-judiciaire-l-usine-famar-lyon-en-pole-position-pour-la-fabrication-de-chloroquine.N945741
Politicians want public money to help FAMAR : https://www.lyoncapitale.fr/actualite/lyon-wauquiez-veut-sauver-famar-seul-fabricant-de-chloroquine-en-france/
I let you guess what political and financial networks led to the disaster of a faked chloroquine study triggering public health concerns.
LikeLike
In the U.K. many landlords are happy to have French tenants as French tenants often think it is a criminal offence if their rent cheques bounce (this is true in France, but not true in the U.K.).
Of course British landlords do not let them into the secret (makes the British chuckle).
https://www.french-property.com/guides/france/finance-taxation/banking/cheques/payment/
“It is a criminal offence in France to write a cheque without sufficient funds in your account, called chèque sans provision.”
Draconian law in France for bouncing cheques, but little to monitor poor scientific practices. That does not seem proportionate.
LikeLike
Pingback: Szyf and Rabbani: old gels evil, new genomics cool – For Better Science
Pingback: The Ballad of Claudio Hetz – For Better Science