Everyone picks on poor Martin Birchall, laryngology professor at UCL in London, regenerative medicine enthusiast, trachea transplanter and former best friend of Paolo Macchiarini. First The Telegraph, and then the BBC brought an online article and two Newsnight stories, where he was portrayed as a kind of person who would bullshit parents into supplying their children as research subjects for dangerous trachea transplant experiments to feed his clinical trial ambitions. People simply do not understand that pigs are not a good model for airway transplants, because pigs tend to die when their tracheas are replaced with a decellularised cadaveric organ seeded with what Birchall decided to call “stem cells“.
And now even those hard-won clinical trials were called off. The funding for EU phase 2 clinical trial with 48 patients was quietly terminated without progressing anywhere, the phase 1 trial it was supposed to be based on indefinitely suspended. It was the clinical trial Inspire with 4 patients, but there is hope: the funder Innovate UK gave Birchall’s partners Videregen a fresh grant to try same technology in a somewhat different setting on 5 patients. Basically, if you are so inclined, a backdoor to somehow revive the suspended Inspire trial. Pity that the patients who died after all those trachea transplants cannot be revived.
RegenVox, terminated
Birchall is a laryngologist, in all his trachea transplanting activities he relied on the help of a thoracic surgeon, to open the patient’s chest and to stick in some cadaveric or plastic trachea coated with patient’s own “stem cells”. Mostly, that thoracic surgeon used to be Macchiarini, for paediatric cases, there were UCL professors Martin Elliott and Paolo De Coppi at Great Ormond Street Hospital. But Birchall is a voicebox and upper airway specialist, there he needs no help operating, and this is why his most favourite clinical trial was RegenVox. Also here cadaveric replacements for larynx and upper trachea were supposed to be used on 10 patients (though Birchall has not given up on plastic scaffolds, as his recent paper indicates). Despite very vocal demands from UCL to lift the suspension, RegenVox was instead terminated by the funder MRC in June 2018.
That RegenVox clinical trial and the data meant to push it forward is what the following story is mostly about, plus there is an issue of misleading representation of a clinical history of a trachea transparent patient from 2010, Ciaran Lynch, whom Birchall operated together with Elliott, De Coppi and Macchiarini.
In December 2012, I published this article, where I reported image reuses in Birchall’s papers and the RegenVox grant proposal. UCL was notified shortly after, and they indeed opened a misconduct investigation. In December 2018, Birchall was acquitted in full and was only asked to issue one minor correction.
I publish the final UCL investigative report here. It is stamped CONFIDENTIAL, but UCL treats everything concerning Birchall as a national secret. The theses of his PhD students are unavailable, Birchall’s testimony in UCL investigation into his and Macchiarini’s trachea transplants is also secret, information about their collaborative deadly trachea transplant from 2010 had to be prized from UCL’s clutches in tiny bits, UCL even refuses to release his press quote originally offered to media in this REF2014 announcement.
There was an earlier report version (to which I then replied), where I also was able to learn the identities of the three internal investigators because UCL didn’t blacken them out properly. The committee’s chair was physicist Robert Speller, neurologist Matthew Walker and zebrafish biologist David Whitmore. None of them has any connection to regenerative medicine, or to airway medicine, or to thoracic surgery, and no external expert was invited to contribute. Apparently, the committee relied on Birchall’s unbiased expertise to guide them through.
The two papers I reported were:
P. Herrmann, T. Ansari, A. Southgate, A. Varanou Jenkins, L. Partington, C. Carvalho, S. Janes, M. Lowdell, P.D. Sibbons, M.A. Birchall
In vivo implantation of a tissue engineered stem cell seeded hemi-laryngeal replacement maintains airway, phonation, and swallowing in pigs J Tissue Eng Regen Med. 2017 Oct 19. doi: 10.1002/term.2596
Ansari T, Lange P, Southgate A, Greco K, Carvalho C, Partington L, Bullock A, MacNeil S, Lowdell MW, Sibbons PD, Birchall MA Stem Cell-Based Tissue-Engineered Laryngeal Replacement. Stem Cells Transl Med. 2017 Feb;6(2):677-687. doi: 10.5966/sctm.2016-0130.
As well as the black-and-white scans of the original RegenVox proposal, available here (note the file “RegenVox3“).
It turned out the authors P. Herrmann and P Lange are the same person: it is the German student Peggy Lange who got married around the end of her PhD graduation, her 2016 thesis itself (supervised by Birchall, on larynx transplants in pigs) will apparently remain unavailable to public for all foreseeable time. UCL namely denied my Freedom of Information request to access that thesis, but I got fragments of the thesis from another source nevertheless, and quote them below.
UCL was also very unwilling to admit that P. Herrmann and P. Lange are the same person indeed, the university even went as far as to mislead me that P. Herrmann was the German ophthalmologist Philipp Herrmann who also did PhD at UCL at exactly same time. After Mr Herrmann denied to me being that person over phone, UCL eventually admitted that Peggy Lange’s new name is indeed Herrmann. In any case, the UCL final report fingered a certain PhD student as sole responsible for image reuse while asking Birchall to be more vigilant with his trainees and co-authors.
The Herrmann nee Lange PhD thesis was excluded from investigation despite my advice. Neither did they get the records of animal experiments from the collaborating piggery, the Northwick Park Institute for Medical Research (NPIMR, authors Tahera Ansari and UCL professor Paul Sibbons are the two bosses there). Those records were namely declared commercially sensitive, so tough luck. Instead, the committee was issued with my article, the PDFs of two papers, and this (highlights mine, “Respondent” being Birchall):
“written responses to the allegations submitted by the Respondent, dated 17 January 2018 and 13 February 2018, that included a copy of the request for Erratum to the Editor of J Tissue Eng Regen Med; a document prepared by the Respondent’s lawyers setting out a summary of his position; a copy of the UCL Special Inquiry report into Regenerative Medicine research at UCL; the operative notes for each of the pigs in the two published preclinical pig studies; written response to the Panel from the former student concerned.”
One step – Two-step – Piggy-in-the-middle
The central confusing issue about those image duplications was that the two papers while re-using data, showed two different methods: a one-step (orthotopic) vs a two-step (heterotopic) approach. In the former (Herrmann et al 2017), the larynx of the pig (or half of it) is removed and a decellularised replacement is put in. In the two-step method (Ansari et al 2017), the graft is first stuffed into the pig’s neck muscle expected to become vascularised and turn into a living organ, and then explanted and used as a replacement for the pig’s own larynx.
We do know the first set of experiments, the one-step transplant, was indeed performed prior to RegenVox funding, since it is described in the proposal, using the same images. There, Birchall experimented on 16 pigs, 8 of which received plastic grafts and 8 of which received cadaveric larynges. The results of the plastic graft experiment were never published, because reasons, but we know that at least one pig died. Of the 8 cadaveric graft pigs, at least 2 animals died, which led Birchall to declare in his RegenVox grant proposal a death ratio of 3 out of 16. Meaning, 8 plastic graft pigs were “revived” and renamed as the cadaveric cohort of happy survivors. None of that was noticed or investigated by the panel when finalising their Report.
There is therefore a reason to doubt the two-step procedure ever was performed as published, what with image duplications and the apparent fact that the Herrmann nee Lange thesis never mentions these experiments. Especially knowing what happened to Birchall’s pigs when same was tried with trachea transplants. Two pigs received orthotopic one-step transplants and responding by dying (read here). The next set of pigs received a heterotopic two-step transplant which proved a resounding success because it was performed only half-way through. The pigs did get a decellularised trachea stuffed inside their neck muscle, but the good doctors never progressed to removing the pigs’ own trachea and replacing it with the allegedly regenerated and vascularised explant. Those lucky porkers were breathing happily through their own unperturbed airways at all times.
Now you wonder what kind of nincompoop one has to be to fall for that kind of experimental cock-up. Well, European Medicine Agency (EMA) not only agreed that all that (i.e., 2 out of 2 dead pigs and 100% mortality ratio, plus at least 8 out of 10 dead patients) proves that the method is safe and reliable, they even granted Birchall’s partners Videregen permission to apply it to children.
Relevant EMA documents available here and here.
Birchall’s version to UCL committee was the following: because his orthotopic one-step method with trachea transplants was a resounding success (cf Claudia Castillo and Ciaran Lynch, other dead patients conveniently forgotten), he for some unfathomable reason decided to abandon it. This was why the two-step heterotopic method has been developed, which unfortunately delivered a high mortality rate since 2 out of 8 pigs died. Good, but not good enough to be used on human patients whose lives are absolutely not in danger and who only consider a transplant to be able to speak. Birchall explained he then tasked Herrmann nee Lange with developing the one-step method de novo, and so he says, all 6 pigs there came out alive and grunting happily. Those 6 two-step pigs were never ever same 6 pigs which participated in the previous one-step ordeal.
That version of course makes no sense to a sane person, but UCL committee loved this one-step-two-step-piggy-in-the-middle game.
Here again is my own reply to UCL’s initial report (which was not much different with the final version) and here is the main section of the “CONFIDENTIAL AND NOT FOR FURTHER DISSEMINATION Research Misconduct Screening Panel” report (I inserted some hyperlinks and illustrations with legends):
Findings of the Screening Panel
Allegation 1
The blog by the Complainant at:
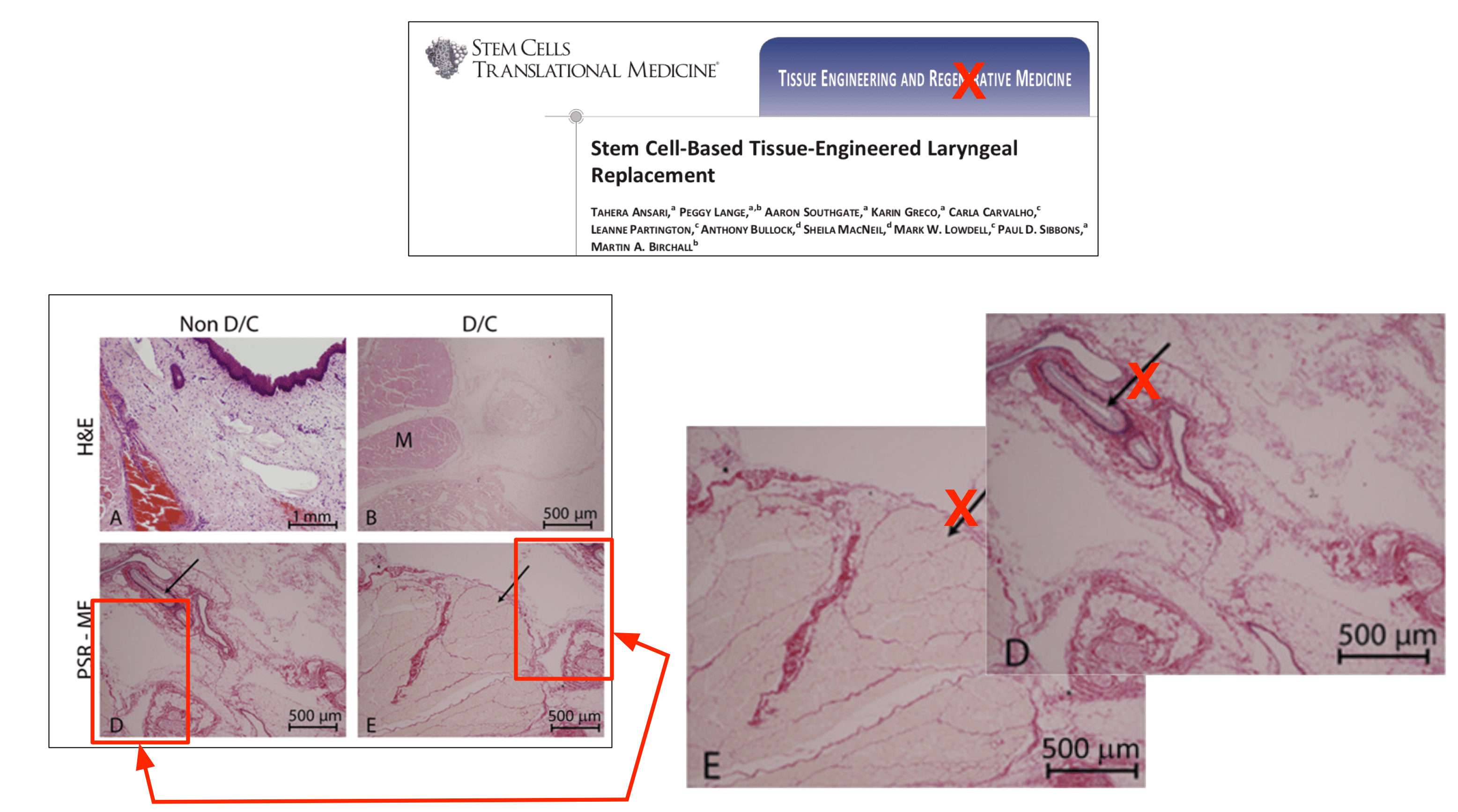
had identified the alleged duplication of images between two articles authored by the Respondent and other colleagues. The blog suggested that some of the images used in Figures, 1, 2 and 3 in the published article, ‘Stem Cell-Based Tissue-Engineered Laryngeal Replacement’ Stem Cells Transl Med. 2017 Feb; 6(2):677-687. doi: 10.5966/sctm. 2016-0130, had been duplicated in a subsequent published article, ‘In vivo implantation of a tissue engineered stem cell seeded hemi-laryngeal replacement maintains airway, phonation, and swallowing in pigs’ J Tissue Eng Regen Med. 2017 Oct 19. doi: 10.1002/term.2596. The blog also suggested that some of the images used in these three Figures had been previously used in a funding application authored by the Respondent for the RegenVOX clinical trial in April 2010.

The Screening Panel found that there had been duplication of images in relation to Figures 1 and 2 in the J Tissue Eng Regen Med article but was satisfied with the Respondent’s written response. The Respondent had noted that Figure 1 related to developmental laboratory characterisation work and that four of the microscopic images presented were duplicates of images published in the previous SCTM article: Figure 1 (a) was also Figure 2B in the previous article, 1(c) was 2(D), 1(d) was 2E and 1(f) was 2F. However, the Panel was satisfied that the existing caption as well as the references to it in the text did not require correction.

The Respondent noted that Figures 2(a)-(c) showed examples of an implant procedure, but were in fact taken from one of the animals referred to in the SCTM article and should not have been included here. The Screening Panel found that Figures 2(b) and 2(C) were the same as Figures 1B and 1E in SCTM but the graph shown in Figure 2(d) was correct. The Panel was satisfied with Professor Birchall’s written response where he acknowledged errors with respect to the images contained in the J Tissue Eng Regen Med article and noted that the duplicated images did not affect the conclusions reached in the article.
The Respondent acknowledged that these errors represented a mistake by the former UCL PhD student concerned and the scientist overseeing the publication. As senior author the Respondent willingly accepted the major share of responsibility for these errors and acknowledged that he should have a kept a closer eye on the preparation of the manuscript before it was submitted.
The Respondent informed the Screening Panel that he had submitted an Erratum request to the Editor of J Tissue Eng Regen Med to publish a correction in relation to Figures 1 and 2 in the TERM article. A copy of the request was provided to the Panel.

In relation to Figure 3, the Respondent had noted that the third and fourth images in Figure 3(b) in the TERM article were the same as Figures A and B in Figure 7c of the grant funding application for the RegenVOX trial. The Screening Panel learnt that in both the article and the funding application the images were clearly labelled as being taken at one month (four weeks) and two months (eight weeks) respectively. Figure 7d (A) of the grant application represented cytology brushings from the laryngeal grafts and was the same as the first image in Figure 3(a) (lower panel). The caption referring to the former did not state a time-point at all, whereas those to B and C stated that the material was harvested at 2 and 4 weeks respectively. A was taken at 4 weeks as presented in the article. The Panel was satisfied with the Respondent’s response in relation to Figure 3 and did not consider that correction was required. It also felt that referring to images used for funding grant applications was not problematic.
In relation to the animal data, the Screening Panel did seek to obtain the Home Office returns and the logbooks of these experiments to determine the outcome for each animal. The Panel was informed that both sets of material contained commercially sensitive material and, moreover, the Home Office returns did not describe each animal but grouped them according to PPL licensee. However, the operative notes for each of the pigs in the studies were supplied and they were the only individual animal record of use except that already published as reviewed papers. The Panel examined the operative notes and was satisfied that the two published preclinical pig studies were separate trials. It also found that all the pigs could be accounted for and that no data had been reused. The Panel concluded that there was a lack of intent to deceive by the Respondent in relation to this allegation.

Allegation 2
The blog by the Complainant at:
https://forbetterscience.com/2017/04/20/ciarans success-story/#comment-10217
had made an allegation of misrepresentation of a patient’s case for the purpose of publishing in a high impact journal and dishonest procurement of public research funding based on this information. The patient concerned was a terminally-ill child who received a tissue-engineered tracheal transplant in 2010 at Great Ormond Street Hospital. The case was reported in the Lancet (2012) and the American Journal of Transplantation (2015). A comment was posted at the foot of the blog by an anonymous writer under the pseudonym “Agrippina” who alleged that (i) there was a discrepancy between the 2012 Lancet article and a brief reference to the same case in a 2011 article in the Annals of Thoracic Surgery describing a series of four patients (including the present patient) receiving bio absorbable stents at GOSH in 2010; and (ii) there was no REC approval for the transplant in this child and that this was inappropriate.

The Screening Panel concurred with the Respondent’s response that the discrepancy between the 2011 and 2012 articles raised by “Agrippina” related to the difference in terminology used in describing the narrowing of the tracheal lumen in the first six months following tracheal transplantation, as well as a marked difference in time scale between the two articles (6 months versus 2 years). The Panel learnt that clinical records described both stenosis and malacia as present/predominant at different times. The Respondent was not an author on the 2011 article but noted that the authors of the 2011 article had used the term “stenosis” to describe the narrowing of the trachea but the team involved in his care, including authors on the 2012 and 2014 articles, were clear that the narrowing of the airway was due to a number of factors and due to a combination of malacia, stenosis, granulation tissue and thick secretions. The 2012 article did not describe the 17 week point where the 2011 article described “severe progressive stenosis”. The treating team were clear that this was a mixture of malacia, granulations and stenosis at this time point.
The Screening Panel learnt that at five months post operatively, both articles agreed on the need for insertion of metal stents. The 2011 article stated that this was due to progressive stenosis whereas the Lancet article states that there was a lack of rigidity. The managing team were clear that the overriding problems were malacia (“floppiness”, “lack of rigidity”) and that this collapse itself was the cause of luminal narrowing described by the 2011 article as “stenosis”. The Panel found that both articles had used different terms to describe the fact that the lumen was compromised. The Panel also observed that both the 2011 and 2012 articles and the subsequent 4-years follow up article published in 2015 were very explicit about the difficulties and airway obstruction faced by the patient in the first six months and about the management required to care for them.
The Respondent confirmed that all appropriate ethical permissions were in place for a formal clinical trial. The Screening Panel found that that there was no requirement for REC approval in compassionate use of medicines, but rather local approvals, and therefore this case was not required to be entered on the UK National REC database. It was noted that there was a difference between approvals required for compassionate use of medicines and those for formal clinical trials. The Panel concluded that there was no attempt to misrepresent the patient’s case by the Respondent in either the published journal articles or in order to obtain public research funding. The need for REC approval was also mistaken.
Conclusions
Having considered the evidence at paragraph 10, the Screening Panel determined that allegation 1 had some substance as there were errors in relation to Figures 1 and 2 in the TERM article. However, due to a lack of intent to deceive by the Respondent, who had already made an Erratum request to the Journal Editor, the Screening Panel concluded that this matter should be addressed through education or training or other non-disciplinary approach rather than through the next stage of the Procedure or other formal proceedings. Therefore, the Panel would make the two recommendations at paragraphs 26 and 27 below.
In relation to Allegation 2, the Screening Panel determined that it was mistaken and that there was no prima facie evidence of misconduct as defined within Annex 2 of the procedure.
Recommendations
That the Respondent liaise with the Editor of J Tissue Eng Regen Med to ensure that the Erratum is published and the corrections are made to Figures 1 and 2 in the TERM article.
That the Respondent takes steps to ensure he checks the detail of manuscripts on which he is an author before they are submitted.
That the second allegation made against the Respondent not be upheld and be dismissed in accordance with paragraph C30 in the UCL Research Misconduct Procedure.

The deadly Lancet
As you can read regarding the 2nd Allegation, airway surgeon Birchall explained to his medically or surgically clueless investigators that tracheal stenosis (narrowing of the trachea) and malacia (softness of tracheal walls) were actually the same thing. And where the Elliott et al 2012 paper blamed the patient’s native bronchi for all the troubles with bronchoscopy, the Vondrys et al 2011 paper admitted that the tracheal transplant collapsed immediately and could only be kept open with a stent. Yet Birchall educated the Committee that it all basically meant the same thing. It was also OK to invent a REC approval which did not exist, because as Birchall explained, it was not necessary anyway.
No hope then to ever see that Elliott et al Lancet 2012 paper corrected. But now The Lancet is under pressure to act on Birchall’s other publication there, the Macchiarini et al 2008 report about world’s first trachea transplant, on patient Claudia Castillo in Barcelona. As I reported before (here and here), what was made to look like a success, was in reality an utter failure: the graft collapsed just 3 weeks later, after years of suffering it was removed together with the patient’s left lung. The medical director of the Hospital Clinic Barcelona, Antoni Castells, sent together with his colleagues a letter to The Lancet to be published, which the journal rejected. It brings no novel insights for the Editor-in-Chief Richard Horton, you see. The issue is now being raised by UK’s Member of Parliament and head of Science & Technology Committee, Norman Lamb, on the BBC:
The 5-year follow-up paper, also in The Lancet, Gonfiotti et al 2014, is an utter work of fiction and everyone knows it. The problem here is that the responsible institution, the Careggi University Hospital in Florence, Italy, prefers to keep omerta and apparently thinks Macchiarini deployed deadly trachea transplants on at least 5 patients there in self defence. After all, Italian court acquitted Macchiarini (of charges of extortion), so Careggi seems to believe in his innocence and that these 4 women (3 of them very young) and one man all deserved to die.
A secret dissertation
As I mentioned before, the PhD thesis of Herrmann nee Lange is top secret. A source however sent me some quotes from that thesis. We learn that there were apparently indeed only 16 pigs, those featuring in RegenVox grant and then revived again for Herrmann et al 2017, and then revived yet again, this time as two-step approach operated, for Ansari et al 2017. On page 202:
“In vivo study larynx replacement
a total of 16 juvenile acclimatised female pigs received implants to reconstruct a surgically created partial laryngeal defects. The animals were randomised in two groups and received either a biological or synthetic scaffold…The use of pigs was justified by the requirement of in vivo studies in large animals, which could be easily translated to human clinical implantation at later stage. Scaffold were placed orthotopically to assess physiological functionality. There are difference in physiology between man and pig, but these differences are less than those between man and other species. Pigs have been previously used successfully for airway research, experience of previous experiments with pigs including detailed preoperative care protocols were available in this group (Birchall et al 2011, Murison et al 2009).”
We learn that “specifically designed bioreactors were developed and manufactured for this study and that “after surgery the planning survival time was 8 weeks”. And then, on page 211:
“All animals survived the initial surgical procedure. Planned termination was 2 months post-surgery. Three animals had to be terminated before their planning termination date. Two pigs experienced respiratory distress (both biological implants, animal 7 and animal 16) and were terminated one week before their planned termination. Post-mortem analysis showed moderate airway stenosis in both animals with evidence of local infectious causes in animal 16 which displayed a chronic abscess formation at the outside of the larynx. The third animal (synthetic scaffold, animal 15) was terminated 2 weeks post-implantation due to endoscopically confirmed scaffold displacement obstructing the airway. At the time of termination the scaffold was found in the stomach at post-mortem examination. All remaining animals showed normal behaviour, breathing and phonation until termination at 2 months. Blood samples taken at 1 week, 2 weeks, 4 and 8 weeks assessing haematology and clinical chemistry showed no abnormalities: white blood cell counts were all within normal range”.

It continues on page 229:
“The first two pigs operated on developed an infective wound site resulting in the area requiring surgical drainage. In the subsequent cases drains were not inserted without negative consequences. There [Three?] of the 16 animals had to be terminated before the planned 8 weeks’ time point. Two presented with inspiratory stridor causing distress at 7 weeks. On post-mortem examination, airway stenosis was noted. Both of these animals had received a biological implant. One of the explanted larynges had a chronic abscess formation on the outside of the thyroid cartilage…The third animal had no respiratory distress whilst undergoing routine endoscopy at 2 weeks; the synthetic scaffold could be seen dislocated and loose in the airway. The animal was terminated and on post-mortem examination the scaffold was found in the stomach. There were limitations to the study: dislocation and even extrusion of scaffolds could have taken place; the animals may have coughed up the material without visible respiratory distress and swallowed it. Excrements were not routinely examined”.
And on page 263:
“in this study survival rates for pigs receiving the synthetic transplant and pigs receiving the biological replacement were similar with 75% for pigs with biological implants and 80% for pigs with synthetic implants. However the number of animals used (8 pigs for each material was too small to draw statistical conclusion out of the pure survival rate…Remarkably none of the implanted non-biodegradable synthetic scaffolds could be identified macroscopically or histologically after explantation at 2 months.
Macroscopically scaffold dislocation, as early as 2 weeks could be observed and histologically after explantation there were tracked neutrophils in the area of implantation leading to the luminal surface of the larynx. One animal with synthetic scaffold that had to be terminated early showed displacement on endoscopy. At post mortem examination the scaffold was found in the stomach. These results may indicate extrusion of the synthetic scaffold possible due to a foreign body reaction as seen in other studies (Cenzi et al 2005).…
However, the decellularized material was also difficult to identify after explantation, though remnants of the scaffold could be found in some animals. This may be due to the degradation of the scaffold. The remnants were located at the outer surface of the larynx.
…Cell fate of the seeded human cells remained inconclusive. As previously stated human epithelial cells could be identified up to 4 weeks after implantation but not after explantation. In 7 of the 8 pigs receiving the biological material and 4 of the 8 pigs receiving the synthetic material these cells could [not?] be found. However all animals displayed a complete epithelial coverage on the operate side. This may also be due to the robust nature of the porcine animal model and rapid ingrowth of host cells from the edges of the defect.
…
It was not possible to draw conclusions concerning the stability of the synthetic material due to the suspicion of complete extrusion. The biological scaffold showed degradation which puts the implant in risk of instability and malaria as seen for trachea replacements (wood et al 2014).”
There were apparently no other RegenVox pigs but these sixteen, hence the image reuse. All transplants failed, the larynx grafts either degraded and swallowed, or they killed the pigs by causing tracheal stenosis. Decellularized implants proved even deadlier than plastic larynges, the latter were never published, the former produced two papers with identical images but different methodology. No two-step experiments were ever apparently mentioned in that PhD thesis. UCL screwed up yet another investigation.
Update 12.03.2020
Luckily Birchall was absolved retrospectively, prophylactically and eternally of all suspicions of data manipulations in Herrmann et al 2017 and Ansari et al 2017. Because there is even more data fabrication:

That was posted on PubPeer in March 2020. And there is more, from a different user:
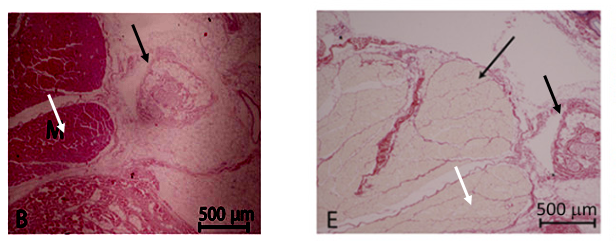
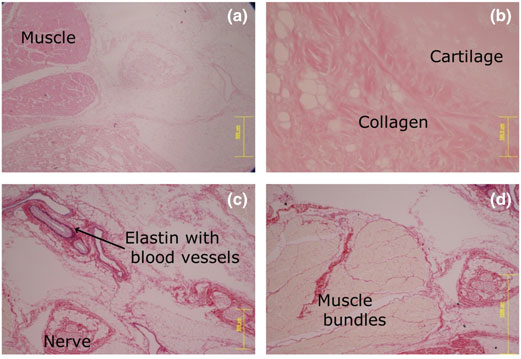
Yet another PubPeer user suspected “Successive slices of tissue, differently stained?” But this cannot be, because those are different samples, different experiments and different pigs! For UCL, this probably only proves that pigs are bad research models and one should rather use humans instead.
In fact, you can call UCL everything, but not quitters. This was published on UCL website on 21 February 2020.
Donate!
If you are interested to support my work, you can leave here a small tip of $5. Or several of small tips, just increase the amount as you like (2x=€10; 5x=€25). Your generous patronage of my journalism will be most appreciated!
€5.00


Birchall shall in the name of her Majesty the Queen be prosecuted to the full extent of UK criminal law.
LikeLike
He is not prosecuted, he gets 300£ per consultation according to Top Doctors website.
LikeLike
I’ll make it my bedtime reading tonight.
LikeLike
Liability exposure + potential gain + fear of bad publicity = always innocent by us.
LikeLike
Hopefully they won’t be able to recruit any patient for the new trial and will have to return the funding
LikeLike
Pingback: The return of David Latchman Show – For Better Science
A corrected version of the following manuscript has recently been submitted:
Herrmann et al, J Tissue Eng Regen Med 2017;1–12
https://onlinelibrary.wiley.com/doi/10.1002/term.2596
The main problem with the original version was that some of the data had been duplicated from a slightly earlier paper by the same group:
Ansari et al, Stem Cells Translational Medicine https://doi.org/10.5966/sctm.2016‐0130
The issue wasn’t so much that the data had been duplicated, but that they were being used to represent two different surgical approaches.
The earlier paper describes a 2-step approach where decellularised-recellularised porcine laryngeal scaffolds were first embedded in the neck muscle, and a month later, implanted into a defect in the larynx.
The Herrmann paper describes a one-step approach where the decellularised-recellularised porcine laryngeal scaffolds were directly implanted into a defect in the larynx.
The duplications raised questions about which procedure had been performed. This was important because the earlier paper was providing supporting data for the RegenVox clinical trial.
Duplicated data appeared in Figs1 and 2 of the original version of Herrmann.
Fig.1 showed microscopic images of sections of the decellularised laryngeal scaffold. Figs 1a, c, d and f have been replaced in the corrected version. Fig. 1e has not been replaced, but this image is actually a bright field image of the original version of Fig.1f (polarised light image). If 2 distinct types of surgery were performed, it would have been necessary to prepare and analyse laryngeal scaffolds for each of the experiments. It is therefore puzzling why the same samples were presented in the two papers in the first place, and why after being corrected, it seems that an image of the same scaffold still appears in both papers.
Fig.2 of the original version showed a macroscopic image of the decellularised-recellularised tracheal graft and an image of it after it had been sewn into the laryngeal defect following the one-step approach. These same images were also shown in the earlier paper that was demonstrating the two-step approach. In the corrected version, the images have simply been removed and replacement images are absent. If images of the different surgeries cannot be provided, this raises questions about whether two distinct procedures were carried out.
This might not seem very important now that MRC funding for the RegenVox trial has been withdrawn. However, the company Videregen has recently been awarded ~£2M to test decellularised-recellularised airway scaffolds in patients with bronchopleural fistula at the Royal Papworth Hospital, Cambridge, UK:
https://gtr.ukri.org/projects?ref=104469
An important question is whether the Herrmann paper is meant to provide supporting data for the Papworth trial.
An FOI request to Innovate UK/UKRI for the list of references that were included in Videregen’s application was denied because it was thought this might disadvantage the commercial interests of Videregen.
LikeLike
Pingback: The Lancet, UNSW and Khachigian's cancer cure – For Better Science
Tenacious investigation of criminal activites at the top of UK Regenerative Medicine. Unfortunately, the wagons have closed and there is no way that UCL will do the right and ‘decent’ thing and end this ghastly affair. Birchall, Sefalian, and others, have benefited financially and career-wise (Sefalian did, until he was scapegoated) from this scandel and the deaths of patients for which the surgeons are culpable.
I amazed that all the clear evidence of fraud, dishonesty, blatant misrepresentation, criminal activity hasn’t led to the governing/regulating authorities taking an interest.
The members of the various ‘whitewashing’ investigatory panels are similarly culpable for the death of the patients because their gutless actions in the face of clear evidence of wrongdoing.
LikeLike
I could not agree more with your last sentence. This point was also made by one of the Swedish journalists, Johannes Wahlström, whose interview can be read here:
https://forbetterscience.com/2017/08/08/the-one-who-asked-questions-interview-with-johannes-wahlstrom-by-alla-astakhova/
Johannes Wahlström says: “Personally, for me as a journalist, the fate of Paolo Macchiarini at some point ceased to be of interest. Much more important is something else. In this story, we saw how hundreds of different people, unconnected in hierarchical order, from different countries, from different areas – from America to Korea, from Russia to Sweden, from journalism to politics and the scientific world – became part of the “conspiracy”. Either they kept silent, or they did not mention it, or they openly lied. But the cumulative result of this “conspiracy” was the death of human beings. Many human beings. How did this become possible? This is the most important question.”
I can provide an update on my comment above about Videregen and the bronchopleural fistula trial.
After my appeal to UK Research and Innovation was ignored, I approached them again, and this time they agreed to provide the reference list (see below). Surprisingly, the pig papers are not referred to, but citations for 3 human cadaveric airway transplants performed under compassionate use are mentioned (references 12-14). It would be interesting to know if these were accurately described in Videregen’s application.
In the meantime, Videregen’s website has been off-line for several weeks. Will the diagrams demonstrating trachea transplants have disappeared when it comes back on-line? These diagrams can be seen here on the previous version of their website: https://web.archive.org/web/20190511041305/http://www.videregen.com/
Also, Videregen’s collaborator, the Northwick Park Institute of Medical Research (NPIMR), has recently renamed itself as ‘The Griffin Institute’: https://griffininstitute.org.uk/. Unlike the old NPIMR website that can be viewed below, the Griffin website no longer boasts that researchers at this institute were involved in a ‘world first’ GMP trachea transplant that was performed on a child patient. The transplant proved to be fatal.
https://web.archive.org/web/20190301083107/http://www.npimr.org/
LikeLike
Prof Murray shared the document with me, and I share it here

LikeLike
Pingback: The English science supremacy – For Better Science