This is a story uncovered and researched with the tremendous help of Swedish investigative journalist, who would like to remain anonymous. A Swedish start-up company, fed with generous funding from Swedish state, now received €2.2 Million from the European Union, to further develop the same regenerative medicine technology which was determined in two investigations to be tainted by research misconduct and patient abuse by the founder of this very company: Suchitra Sumitran-Holgersson. A clinical trial is scheduled based on debunked science of recellurising dead blood vessel grafts, to continue where 3 previous child patients were used as human Guinea pigs .
This might remind you of another ongoing Horizon 2020 clinical trial, where a similar decell-recell technology of the trachea transplanter Paolo Macchiarini is being prepared for mass-use in patients. There, EU refused to share any information. Also in the case of Sumitran-Holgersson and her company, all EU commission members and even press speakers I approached refused any communication.
A company formerly known as NovaHep
Things seem to go very well for a small biotech company in Gothenburg, Sweden. In June, VeriGraft was granted almost 2,2 Million € from Horizon 2020, the biggest EU research and innovation programme. This is no minor achievement. Out of the 1222 European applicants, only 69 got funded and VeriGraft was one of only nine life science companies. One might wonder what kind of groundbreaking research was exciting enough for the Horizon 2020 experts to grant one of their biggest sums to a company with just ten employees. The funding is to be used to commercialise their tissue-engineered veins, with a clinical trial as a crucial stepping stone. There is no doubt that the EU experts saw great potential in VeriGraft and their vein product. But why? Here’s the story of the company and its core research.
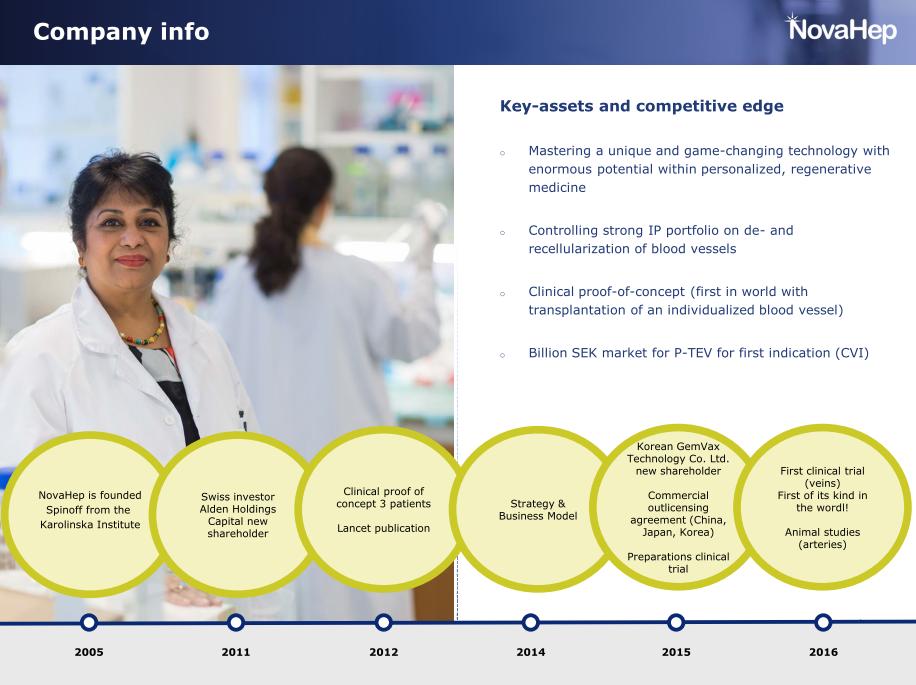
The scientist behind VeriGraft (formerly NovaHep): Suchitra Sumitran-Holgersson. Now for some reason hidden from the spotlight. Source: NovaHep
VeriGraft was founded as NovaHep in 2004 by Transplantation Biology Professor Suchitra Sumitran-Holgersson together with her then-husband, Professor Jan Holgersson, while both were employed at the Karolinska Institutet (KI) in Stockholm. The KI spinoff was developing hepatic stem cells to the pharmaceutical industry. In 2008, the company moved to Gothenburg, as its founder Sumitran-Holgersson was recruited by Michael Olausson, Professor in Transplantation Surgery, to Gothenburg University (GU).
The regmed geniuses of Gothenburg
Sumitran-Holgersson’s and Olausson’s research groups worked closely together at Sahlgrenska Science Park, a platform meant to promote collaboration between academia, healthcare and business. This collaboration resulted in four transplantations where patients received decellarised donated organs, recellularised with their own cells. The following years, news about the tissue-engineered transplantations in Gothenburg were announced as scientific breakthroughs all over the world. A girl received a decellularised vein recellurised with bone marrow stem cells! A donated trachea was regenerated and implanted onto an elderly patient (who unfortunately, died soon after). And then, two more children were said to have undergone successful transplantations with donated veins, tissue-engineered with an even more groundbreaking technique. Sumitran-Holgersson produced the grafts and Michael Olausson performed the surgeries, the Gothenburg University described this as “Stroke of genius”. Studies were published, in The Lancet, Tissue Engineering, and EbioMedicine. For the last two cases, it is described how only 25 ml of the child patients’ own peripheral whole blood, containing maybe a dozen of circulating endothelial cells, was used to regenerate the donated decellurised veins with living, functional vessel walls in just ten days. Just 10-12 endothelial cells in 25 ml of blood, doing all this, an impressive feat of biology; much more advanced compared to the heart valve regeneration technology inside the patient’s body, which was developed by the German heart surgeon Axel Haverich.
This technique later became the basis of VeriGraft’s new main product, “personalized tissue engineered veins” (P-TEV). Quickly and easily made host-compatible veins with one goal market in the huge patient group with chronic venous insufficiency. The method was patented by Sumitran-Holgersson and Olausson in 2015 and sold to VeriGraft. The articles about the three girls transplanted with recellularized grafts were, and are still, used as “proof of concept” for VeriGraft’s product claims, always mentioned to convincingly apply for grants and attract investors, for example here.
The story of how these same scientists were later critiqued, investigated, found guilty of scientific misconduct and that Sumitran-Holgersson is still under investigation for scientific fraud in up to ten publications, has been accounted for here and here. Incidentally, Sumitran-Holgersson left her post as head of research at VeriGraft around the time that GU initiated their investigations. She and Michael Olausson have royalty-agreements to get their shares of future profits.
Guilty of misconduct
As reported here, Sumitran-Holgersson’s recruiter, and later partner in scientific misconduct, Olausson, had been of indispensable assistance in getting her out of previous scientific fraud accusations around the time of her move to GU. A report from the then-Swedish investigative authority, Vetenskapsrådet, in 2009, stated that they found “several cases of data fabrication” and that “Holgersson’s research lacks critical thinking and a sound scientific approach”. This investigation and its results were later dismissed for formality reasons, while the two investigators were threatened with lawsuits by Jan Holgersson’s other company, AbSorber.
Both Sumitran-Holgersson and Olausson were eventually found guilty of research misconduct for their experiments on child patients, where they were caught lying in their publications about the existence of ethics approvals. This is well documented by GU’s internal review (here and here) as well as the external investigation (full report here) led by Bengt Gerdin and Kjell Asplund, two clinicians who previously investigated another Swedish scandal of regenerative medicine: that of the former KI professor and trachea transplant surgeon, Paolo Macchiarini. Incidentally, it was then Olausson who advised Swedish state prosecution to drop manslaughter charges against Macchiarini in October 2017. Indeed Olausson is a recognised expert in patient abuse, in fact even an official Lex Maria note was sent by GU to the Swedish authorities notifying of potential abuse of patients, by Sumitran-Holgersson and Olausson. In May 2017, Sumitran-Holgersson demanded that the more recent, and still ongoing, GU investigation of data manipulations in ten of her articles was to be sent to the Swedish Central Ethical Review Board (CEPN). The CEPN report is to be finalized now in January 2018, almost two years after the complaint was first reported to the GU principal.
Non-Compassionate Use
Meanwhile, VeriGraft has continued to receive grants and investments to proceed with the commercialization of their vein product, all the while leaning on their published proof-of-concept of three successful cases. It is worth discussing here what “successful” means in context of such individual patient treatments. Obviously, the scientific results passed scientific peer review and were successfully published in elite journals like The Lancet and its spin-off EBioMedicine. But so were the human experiments of Macchiarini, like his lethal research with plastic trachea on an Icelandic patient, which also became a paper in The Lancet. That trachea transplant therapy, just as the three vein transplantations by Olausson and Sumitran-Holgersson (and their own trachea transplant), were declared to be so-called “compassionate use” cases, with the purpose of saving lives of dying patients using unconventional therapies. By declaring this to not be research, Macchiarini and the team Olausson/Sumitran-Holgersson effectively weaseled themselves out from obtaining proper ethics and medicinal product approvals. With compassionate use, scientific interests can never be the prime goal of intervention. Success of compassionate use interventions is therefore not measured on the impact factor of the journal where the results were published, but on the life extention or life quality improvement of the patient. There is little reason to assume the 3 children really benefited from the VeriGraft vein transplants, but the scientists and businesspeople behind those certainly did.
As for the VeriGraft technology poised to enter a EU-funded clinical trial: only two of these three patients received grafts produced with that technique, namely regenerated with 25 ml of peripheral whole blood. The first child was repeatedly transplanted with vein grafts recellurised with bone marrow cells, a technology similar to that was used by Macchiarini as well as the Olausson/Sumitran-Holgerson team for trachea transplants.
In fact, two of the three grafts seeded with peripheral whole blood failed post implantation, at least one of them due to occlusion, as described in Olausson’s and Sumitran-Holgersson’s EBioMedicine article. One of these two children, at the time 22-months old, had to be saved with a liver transplant. This results in one potential proof-of-concept transplantation, and the Gerdin report describes the clinical situation of that child patient as follows:
“In the medical records it is reported that the meso-Rex bypass still functions, 4 years after the transplantation, but that the patient still suffer from portal hypertension and splenomegaly. The patient needs to take anticoagulants (Waran) and will probably need lifelong specialist medical care”
Since the GU misconduct reports, VeriGraft stopped describing the three patients as “very healthy”, as all three girls will need lifelong medical care, but they still refer to all three cases as successful, seemingly based on the notion the results were successfully published and that the patients were not given any immunosuppressive medications. It is the same line of argument Macchiarini uses.

Debunked in Norway
Since 2013, there have been plans for a phase 1 clinical trial with VeriGraft veins at Oslo University Hospital in Norway, and a VeriGraft sponsored study to clinically test the technique with peripheral whole blood on patients was approved by the Regional Ethical Review Board (REK) in February 2016. After the initial application, REK requested more information (see file here). Among other things, REK found the medical justification and the potential risks for the patients in the study insufficiently covered. The committee was concerned about what alternatives the patient would have if the implanted vein segment did not work, or in a worst case scenario, was rejected. They wanted these questions evaluated by a specialist in vascular surgery before proceeding. Once again, the proof-of-concept cases worked in VeriGraft’s favor. The committee was soon assuaged by the consulted specialist Jon Anders Torbjørn Jonung, who in his “medical motivation” stated:
“there are two publications where children […] have been treated with a bypass with a tissue-engineered vein. The vein was manufactured by the same company that is in this application. There were no signs of infection. The vein functioned properly”.
Thus, the ethical application for the phase 1 clinical trial in Oslo got its final approval. It wasn’t until 2017, after vascular surgeon Antonio Rosales had taken the role as the chief researcher of the Norwegian study, that measures were taken to control the accuracy of VeriGraft’s research claims (see full Rosales report here, there’s a manuscript draft in English, pages 258-271 ). It was decided that there was a need to pre-clinically validate the study process,
“since the theoretical background on the prevalence of blood ECs [endothelial cells] does not fully integrate with the positive results on blood re-endothelialization published in the Olausson et al. study”.
Rosales requested permission from REK to conduct an in vitro study of the P-TEV grafts produced by VeriGraft. The results were sent to REK in October 2017: The grafts did not contain any endothelial cells and thus couldn’t be used in a clinical study. It is concluded:
“In contrast to the proof of principle publication, we could not find evidence for recellularization of the decellularized scaffold, and did not observe the formation of a new endothelial layer.”
The study also suggests an explanation to how someone would mistakenly get the impression that the scaffolds are re-endothelialised. Two established immunohistochemical staining procedures to detect the presence of endothelial cells (for CD31 and von Willebrand factor (vWF)) gave positive results when VeriGraft’s whole blood recellurisation technique had been used, even when no such endothelial cells were actually physically present on the graft. In fact, vWF staining was positive even on decellurised, cell-free grafts prior to recellurisation. More importantly, this effect is not just a laboratory artefact, but potentially dangerous, as a naked vessel wall can predispose to thrombosis. The presence of vWF, platelets and fibrin along the lumen of the vessels was confirmed.
VeriGraft has now gone from describing their method as “recellularization” to “reconditioning/recellularization” on their website and seems to put a little less emphasis on the proof-of-concept articles and more on yet unpublished animal studies. The Norwegian research group however has canceled their collaboration with the company. The EU is still spending €2,2 MIO to develop a product that doesn’t appear to live up to its most crucial claim, the unique regeneration of a dead vein graft with peripheral whole blood.
More money is coming in. What with the resounding success in patients with veins, VeriGraft now received public funding to grow arteries using same technology, for the collaborative project “Personalized Tissue Engineered Arteries (P-TEA) as a Novel Cure for Cardiovascular Disease”. With such pseudo-regenerated arteries however, the patients’ main risk will not be thrombosis or organ failure. It is very likely to be massive internal haemorrhage, and immediate death.
Update 3.01.2019. The clinical trial “Tissue Engineered Veins in Patients With Chronic Venous Insufficiency (TECVI-1)” has now been registered on ClinicalTrials.gov. on 21 December 2017, incidently on exactly the same day as Swedish authorities forbade the University of Gothenburg to sack Sumitran-Holgersson. No location or contacts named yet.
Donate!
If you are interested to support my work, you can leave here a small tip of $5. Or several of small tips, just increase the amount as you like (2x=€10; 5x=€25). Your generous patronage of my journalism will be most appreciated!
€5.00


Thank you for sharing this interesting text. I am working with my first dokument for pubpeer. I hope I have it ready soon and that it is easy to read. What you are doing is brilliant. As soon as I get fundings again I am interested in supporting you because I know the importance of what you are doing. I have only threats about legal actions around me. I understand that you are in the middle of them. That is awful. You should get payed instead. Because you fight for science and so do I.
Kind regards Monica
LikeLike
Olausson tried to save Macchiarini from criminal investigation and Macchiarini had to pay back. May be he was the one who reviewed EU grant applications? Or may be he only provided his best reccomendations while it was Birchall, Jungebluth and their friends who approved this project? Once the closed circle of reviewers is formed, they can grant to each other money for whatever nonsense. But to give funding to someone who is under investigation for fraud? Hm…. May be that is one of the required merits? Need to check call for proposals….
LikeLike
Thanks for a very interesting update regarding Sumitran-Holgersson.
I have been waiting for a conclusion from the investigation which has taken too long,
and was about to contact GU/CEPN again.
I was really surprised to hear that REK in Norway approved the clinical trial.
This illustrates again that we need stronger measures to stop unethical behavior and
fraud in biomedical research and avoid results based on false data entering clinical trials.
In sports the cheaters are banned from participating, but in biomedical research they
are often protected and can continue with minimal or no consequences. There are no
independent international research institutions that can act as judges and follow the necessary
legal procedure to stop researchers from doing research misconduct. ORI in the US is just a makeup (seven misconduct cases reported in 2016 and 2017) and EU’s policy is very nicely exemplified in your article.
LikeLike
An interesting “Science Blog: The Truth in Science” here (http://tbud.de/), posted in April 2017 and educating about NovaHep. Quote:
This unbiased, utterly objective post appeared unsigned on a website which domain is registered (according to denic) to Raimund Strehl from Regensburg. Same Strehl is as indicated above, CTO of VeriGraft/NovaHep.
Btw, Strehl also co-wrote a book, in German: “Zukunftstechnologie Tissue Engineering: Von der Zellbiologie Zum Kunstlichen Gewebe”
LikeLike
Pingback: Boletim de Notícias: Mesmo após Mariana, abusos da mineração prosseguem em MG | Direto da Ciência
What is a “Temporary removal”?
https://pubpeer.com/publications/BB5A1D66CF6284A0610BC29F48A837#4
LikeLike
A Vatican-approved form of birth control?
LikeLike
Pingback: Sumitran-Holgersson and Olausson to retract 8 papers for research misconduct – For Better Science
Pingback: EU trachea transplant clinical trial TETRA “uncertain to take place” – For Better Science
Pingback: Gothenburg to sack Sumitran-Holgersson, requests 7 retractions – For Better Science
Pingback: Münster charges Jens Schwamborn’s mentor Andreas Püschel with research misconduct, again – For Better Science
Pingback: Cold Fusion by EU Commission: a Fleischmann-Pons revival – For Better Science
Pingback: The first uterus transplant, or how Lancet apologised to Saudis – For Better Science